Week in Regulation
January 4, 2021
2020 Ends on a Modest Note
Following a series of preceding weeks with either costs or savings in the billions of dollars, the last week in regulatory action brought a relatively muted haul to cap off 2020. A Department of Health & Human Services (HHS) action that made a series of changes to Medicare and Medicaid – including some pertaining specifically to COVID-19 – was the most notable rulemaking of the week. Across all rulemakings, agencies published $193.7 million in total net costs and added 554,201 hours of annual paperwork.
REGULATORY TOPLINES
- Proposed Rules: 25
- Final Rules: 58
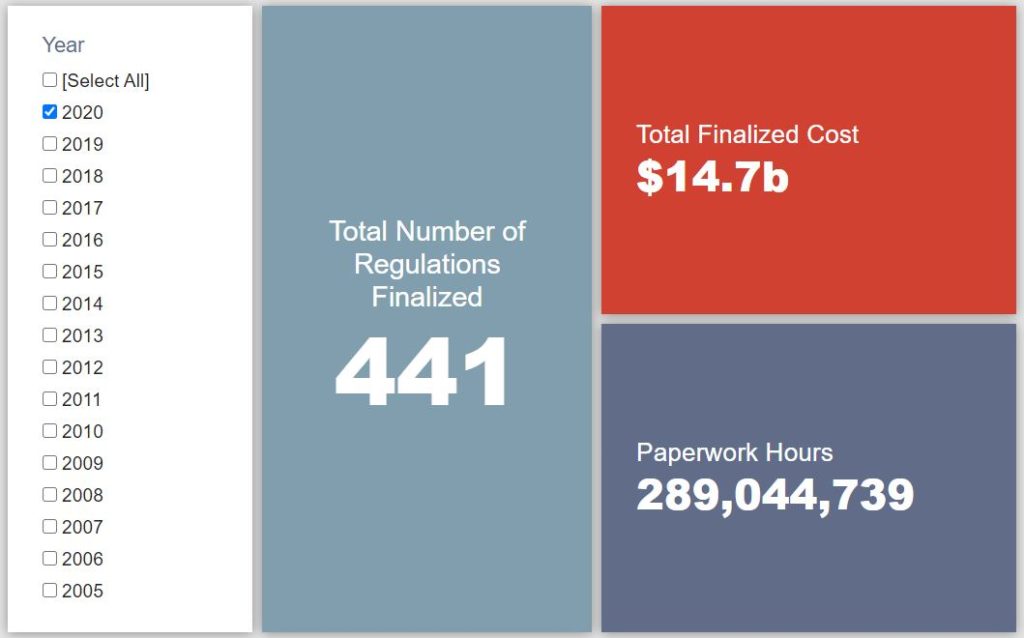
- 2020 Total Pages: 87,248
- 2020 Final Rule Costs: $14.7 billion
- 2020 Proposed Rule Costs: $20.5 billion
TRACKING THE REGULATORY BUDGET
The most significant rulemaking of the week with regards to the fiscal year (FY) 2021 regulatory budget was the aforementioned HHS rule. The rule, which has a far too cumbersome title to include here, makes more than a dozen changes to various parts of Medicare and Medicaid. Among the various topics covered, the measure
Establishes new requirements in the hospital and critical access hospital (CAH) Conditions of Participation (CoPs) for tracking COVID-19 therapeutic inventory and usage and for tracking the incidence and impact of Acute Respiratory Illness (including, but not limited to, Seasonal Influenza Virus, Influenza-like Illness, and Severe Acute Respiratory Infection) during the ongoing COVID-19 PHE [Public Health Emergency].
Despite the wide breadth of topic covered, however, the rule’s impact is relatively mild as compared to some other Medicare and Medicaid rules. HHS estimates that, for regulatory budgeting purposes, it will only impose roughly $7 million in annual costs (or $100 million in present value).
While the administration’s FY 2021 regulatory budget caps are still forthcoming, so far into FY 2021 agencies have officially published 63 deregulatory actions and 18 regulatory actions (as defined by Executive Order (EO) 13,771), totaling $35.4 billion in quantified total net costs. It is worth noting at this point, however, that regardless of whether or not the current administration releases said caps, the Biden Administration assumes power in January 2021. It is highly unlikely that EO 13,771 will remain operative (at least in anything resembling its current form) beyond then, and thus the FY 2021 regulatory budget “window” will be a truncated one. Nevertheless, the American Action Forum (AAF) will continue to track EO 13,771 activity through the end of the administration to provide a record of the regulatory budget initiative’s historical legacy and implications. AAF’s review of the administration’s FY 2020 regulatory budget progress can be found here.
THIS WEEK’S REGULATORY PICTURE
This week, the Federal Aviation Administration (FAA) issues new rules on drones.
 Source: Photo by Jared Brashier on Unsplash
Source: Photo by Jared Brashier on Unsplash
On December 28, the FAA announced that it had finalized two rules that it says will “support technological and operational innovation and advancements” in the use of unmanned aircraft (UA), more commonly known as drones.
The first rule requires drones to have remote identification capability in order to be licensed to operate. Under the rule, all drones required to be registered with the FAA must be able to broadcast a signal with information identifying the device. The FAA hopes the rule will allow drones to operate more seamlessly with existing air traffic. The rule allows existing drones without broadcast capability to broadcast modules to comply with the new requirement and also permits a drone registered in a foreign country to be operated in the United States if the operator files a notice with the FAA. The agency made some changes in the final rule from its proposed version, including scrapping a requirement that transmissions be network or internet based. Only broadcast signals are required in the final rule.
While the rule will go into effect 30 days after it is published in the Federal Register, there are two separate compliance dates. Drone manufacturers must comply with the production requirements within 18 months, while operators will have an additional year to achieve compliance. The FAA expects the lifetime costs of the rule to reach more than $185 million.
The second rule sets the standards for letting drone operators use UAs over crowds and at night. Operators must pass a test and undergo recurrent online training to be permitted to fly drones at night. The regulations for operation over crowds are a bit more complicated. The FAA separates drones into four categories based on the weight and type of drone, but all categories must not have rotating parts on the outside of the drone that could lacerate skin if it were to strike people in a crowd. In the proposed version, the lightest drones were permitted to have rotating external components, but that has been revised in the final rule. The new rule will go into effect 60 days after it is published in the Federal Register, likely early March. The FAA expects regulatory cost savings of more than $550 million over the life of the rule.
TOTAL BURDENS
Since January 1, the federal government has published $35.2 billion in total net costs (with $14.7 billion from finalized rules) and 355.3 million hours of net annual paperwork burden increases (with 289 million hours due to final rules). Click here for the latest Reg Rodeo findings.