Week in Regulation
July 19, 2021
A Surprising Regulatory Bill
Last week’s regulatory haul represented the not-uncommon pattern of a single rule dramatically overtaking an otherwise underwhelming week. But for this one rule, the week’s total would have been $14.7 million in costs with roughly 54,000 hours of paperwork. An interim final rule (IFR) regarding “surprise billing” practices, however, changed that. Across all rulemakings, agencies published roughly $10.6 billion in total net costs and added 6 million annual paperwork burden hours.
REGULATORY TOPLINES
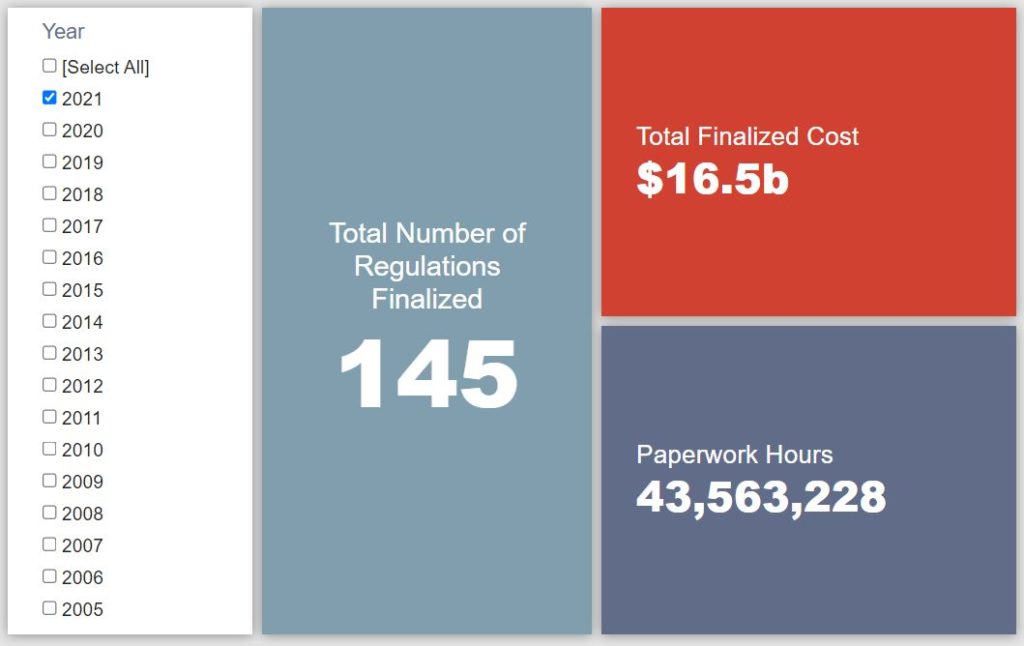
- Proposed Rules: 21
- Final Rules: 75
- 2021 Total Pages: 37,826
- 2021 Final Rule Costs: $16.5 billion
- 2021 Proposed Rule Costs: -$13.4 billion
NOTABLE REGULATORY ACTIONS
The most consequential rulemaking of the week was far and away the joint IFR from the Departments of Health & Human Services, Labor, and Treasury as well as the Office of Personnel Management (collectively, “the agencies”) implementing provisions of the No Surprises Act, which seeks to address the practice of “surprise billing.” Broadly speaking, the issue in question involves patients receiving “out-of-network” bills for treatments or services they expected to be covered “in-network.” Further context on the practice generally and the Act specifically can be found here.
While the agencies cannot provide a definitive, quantified benefit estimate, the rule’s analysis does discuss how alleviating these bills – often in the tens or hundreds of thousands of dollars – is an important goal. The rule’s analysis also lays out how complicated, and thus administratively costly, of an issue this is to fix. For one, it of course involves the policies of four different federal agencies. It also must navigate the myriad connections between state-level authorities and private actors in providers and insurers. The agencies estimate that, over a five-year window, the costs to all involved will be roughly $10.6 billion, with approximately 5.9 million hours of new annual paperwork being a large component of that sum.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the new administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
![]()
The Surprise Billing rule certainly left its mark on the Biden Administration’s to-date regulatory tally. The nearly 6 million hours of paperwork from that rule further widened the gap between the current administration and its predecessors on that front. In the cost column, however, despite that rule’s singular impact, the Obama total outpaced the Biden sum by an even more dramatic margin. The reason for that was a Department of Energy rule on efficiency standards for fluorescent lamps that imposed nearly $20 billion in costs – incidentally the highest cost total of any final rule in 2009.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) further delays the effective date of a 2020 cigarette packaging rule.
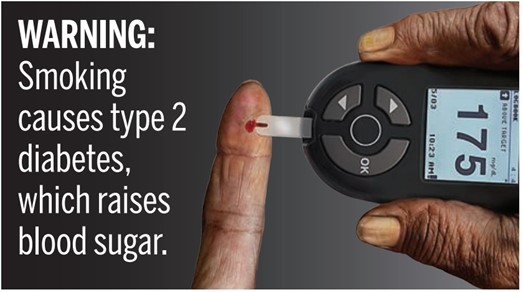
On July 13, the FDA published a third delay of its March 2020 rule implementing a part of the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act) of 2009. That rule required manufacturers of cigarettes to include any of 11 warnings with graphic depictions of the negative health effects of smoking, one of which is pictured above.
The labeling requirements were originally scheduled to go into effect June 18, 2021. That date had been extended twice, however. In May 2020, the FDA extended that deadline to October 16, 2021, and in January of this year pushed the deadline to January 14, 2022. This latest extension moves the date to July 13, 2022.
All of the extensions are court mandated, stemming from a legal challenge to the rule filed in April 2020 that the warnings violate the First Amendment. The merits of the case have yet to be decided, and the extensions mostly are from delays caused by COVID-19.
The March 2020 rule is the FDA’s second attempt at issuing a graphic warning rule under the Tobacco Control Act. In 2011, the FDA published a final rule that required one of nine graphic warnings to be included on cigarette packaging. Those warnings were more disturbing than the 11 warnings required by the new rule, and in 2012 the U.S. Court of Appeals for the D.C. Circuit held that it violated the First Amendment, sending the FDA back to the drawing board.
TOTAL BURDENS
Since January 1, the federal government has published $3.2 billion in total net costs (with $16.5 billion in new costs from finalized rules) and 31.9 million hours of net annual paperwork burden increases (with 43.6 million hours in increases from final rules).