Week in Regulation
September 26, 2016
Energy Efficiency Regulation Drives the Week
For the second consecutive week, the Department of Energy (DOE) published a notable efficiency regulation. This drove total costs for the week to $9.2 billion, with $624 million in annual burdens, and $1.6 billion in benefits; regulators published one million paperwork burden hours. Health and Human Services (HHS) published the only other notable rule of the week. The per capita regulatory burden for 2016 is $481.
Regulatory Toplines
- New Proposed Rules: 52
- New Final Rules: 73
- 2016 Total Pages of Regulation: 65,852
- 2016 Final Rules: $106.2 Billion
- 2016 Proposed Rules: $49.6 Billion
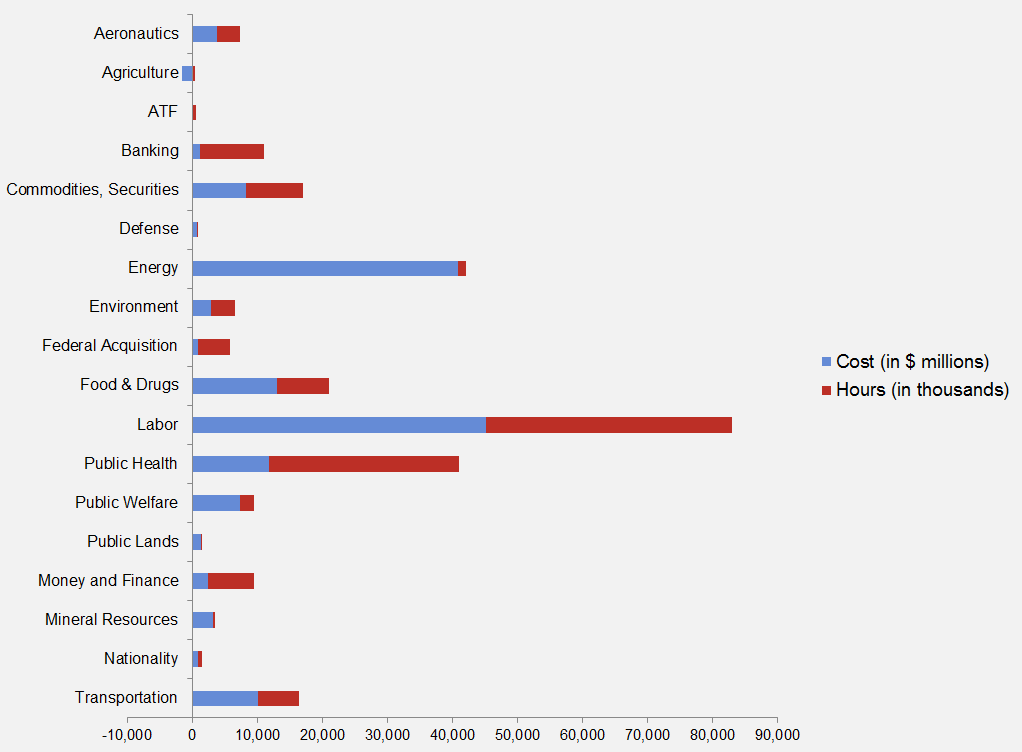
The American Action Forum (AAF) has catalogued regulations according to their codification in the Code of Federal Regulations (CFR). The CFR is organized into 50 titles, with each title corresponding to an industry or part of government. This snapshot will help to determine which sectors of the economy receive the highest number of regulatory actions.
DOE proposed a set of energy efficiency standards for residential furnaces this week. This is actually the second proposed rule for furnaces; the agency published a version in 2015, but amended the rulemaking after it was encouraged to create a separate product class for small furnaces. The 2015 proposal imposed $12.3 billion in net present value burdens and $741 million in annual costs. This recent revised proposed rule lowers those totals to $9.2 billion and $540 million. Despite the drop in costs, the benefits of the revised regulation are actually higher: from $1.5 billion to $1.6 billion. Regrodeo.com, AAF’s rulemaking database, only tallies final rules, so these two proposals are not double-counted online.
HHS finalized a measure to establish a submission process for clinical drug trials. The rule is designed to help patients find drug trials that might aid in their treatment and prevent unsafe trials. The measure will cost $59 million annually. As HHS notes, “The costs consist primarily of the time needed to organize, format, and submit to ClinicalTrials.gov information that was prepared for or collected during the clinical trial.” The paperwork burden is slightly more than one million hours.
Another HHS paperwork notice pushed cumulative reporting and recordkeeping requirements to 11.4 billion. A collection for patient privacy went from 32 million hours to more than 921 million hours. The agency reports that this costs nothing, but a review of the supporting docket suggests more than $57 million in annual costs.
Affordable Care Act
Since passage, based on total lifetime costs of the regulations, the Affordable Care Act has imposed costs of $48.5 billion in final state and private-sector burdens and 171.4 million annual paperwork hours.
Dodd-Frank
Click here to view the total estimated revised costs from Dodd-Frank; since passage, the legislation has produced more than 74.8 million final paperwork burden hours and imposed $36.3 billion in direct compliance costs.
Total Burdens
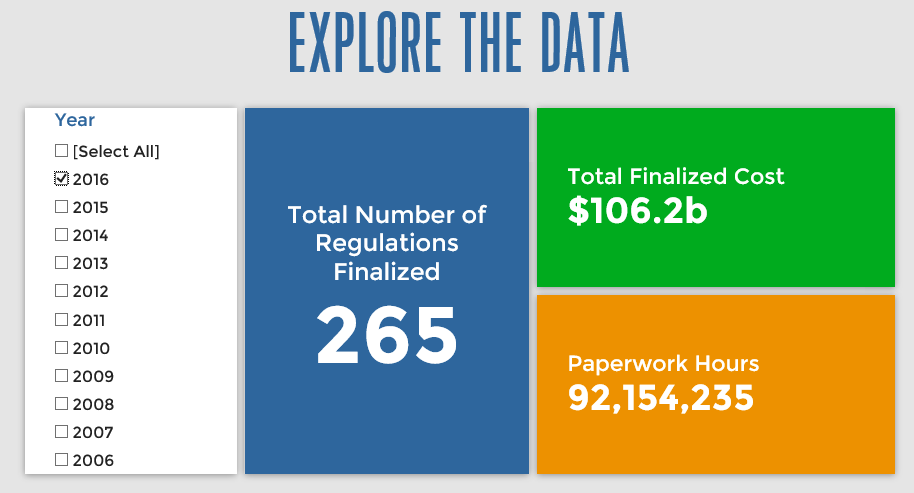
Since January 1, the federal government has published $155.8 billion in compliance costs ($106.2 billion in final rules) and has imposed 127.7 million in net paperwork burden hours (92.1 million from final rules). Click below for the latest Reg Rodeo findings.