Week in Regulation
October 18, 2021
SEC Proposal Stands Out in Otherwise Ho-Hum Week
Coming off a conspicuously busy week from the one preceding it, last week was generally looking like a let-down in terms of measurable regulatory activity. If not for one rulemaking that hit the books on Friday, last week’s regulatory haul would have had little to remark on. A proposed rule from the Securities and Exchange Commission (SEC) provided the bulk of the week’s regulatory burdens. Across all rulemakings, agencies published $373.7 million in total net costs and added 253,414 annual paperwork burden hours.
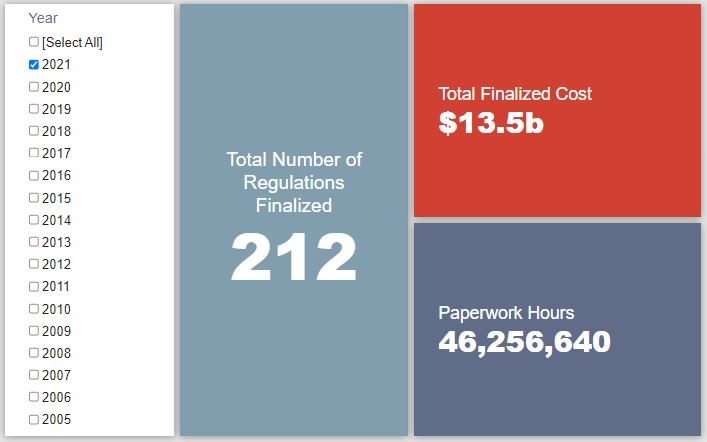
REGULATORY TOPLINES
- Proposed Rules: 27
- Final Rules: 30
- 2021 Total Pages: 57,429
- 2021 Final Rule Costs: $13.5 billion
- 2021 Proposed Rule Costs: $186.1 billion
NOTABLE REGULATORY ACTIONS
The most significant action of the week was the SEC proposed rule regarding “Enhanced Reporting of Proxy Votes by Registered Management Investment Companies; Reporting of Executive Compensation Votes by Institutional Investment Managers.” The proposal seeks to update an SEC form in order “to enhance the information mutual funds, exchange-traded funds (ETFs), and certain other funds currently report annually about their proxy votes and to make that information easier to analyze.” SEC estimates that the changes it is pursuing in this rulemaking would increase the annual paperwork burden for affected entities by nearly 250,000 hours. Given the complicated – and thus expensive – information involved, this translates to nearly $94 million in annual regulatory costs, or $281 million total over the 3-year window that the paperwork requirement would be in place.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the new administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
![]()
Since the primary action from last week came on the proposed rule side, there was little change in the Biden Administration’s final rule tallies. In fact, there was only one area across the covered administrations that saw a notable shift: the Trump-era paperwork total. That figure saw an uptick in mid-October 2017 of more than 700,000 annual paperwork hours. The main driver of this shift was a Federal Communications Commission action regarding copper loops that imposed roughly 575,000 annual hours of paperwork.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) announces an end to an emergency hand sanitizer production policy.
 Source: Photo by Kelly Sikkema on Unsplash
Source: Photo by Kelly Sikkema on Unsplash
In the October 13 edition of the Federal Register, the FDA published a notice that it was withdrawing guidance that enabled the production of alcohol-based hand sanitizers by non-drug manufacturers in response to the COVID-19 pandemic. The notice specifically withdraws three separate guidance documents: one for the preparation, one for the compounding, and one for the manufacture of hand sanitizers.
The FDA issued the guidance documents in March 2020 during a surge in demand for hand sanitizers during the onset of the COVID-19 pandemic. The guidance promised not to enforce regulations that barred non-drug manufacturers from producing alcohol-based sanitizers if those manufacturers met the requirements within the guidance.
In withdrawing the three guidance documents, the FDA explained that even though the pandemic is still ongoing, traditional manufacturers have increased their supply, demand has decreased, and most consumers and health care personnel are no longer struggling to find access to sanitizers. In addition, some of the products made by non-drug manufacturers “have been recalled, placed on import alert, and/or been the subject of compliance actions due to quality and other issues.”
Non-drug manufacturers producing sanitizer are not out of luck yet. The FDA will allow these companies to continue to manufacture sanitizer through December 31, 2021, and sales and distribution to continue through March 31, 2022.
TOTAL BURDENS
Since January 1, the federal government has published $199.6 billion in total net costs (with $13.5 billion in new costs from finalized rules) and 52.6 million hours of net annual paperwork burden increases (with 46.3 million hours in increases from final rules).