Week in Regulation
November 23, 2020
Significant Immigration Proposal Leads Net-Regulatory Week
While it was a relatively muted week in terms of the volume of activity, there were some rulemakings with notable effects cutting in both directions on the regulatory ledger. The most significant action was a Department of Homeland Security (DHS) measure that could bring billions in costs, although it is unclear if there’s truly a path to it becoming final before the end of the Trump Administration. Across all rulemakings, agencies published $6.7 billion in total net costs and added 2.4 million hours of annual paperwork.
REGULATORY TOPLINES
- Proposed Rules: 38
- Final Rules: 69
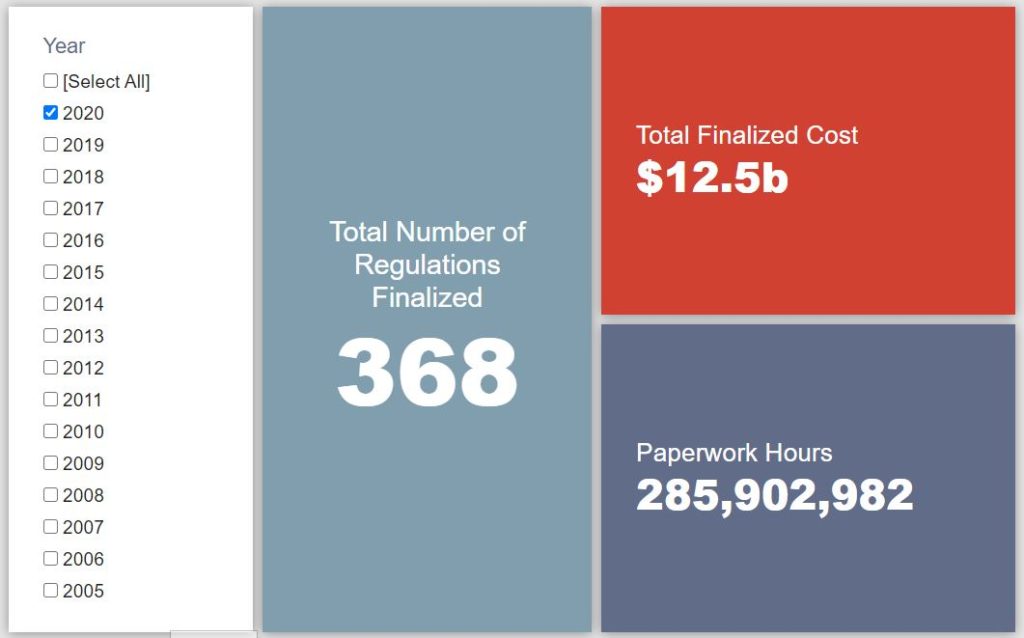
- 2020 Total Pages: 74,502
- 2020 Final Rule Costs: $12.5 billion
- 2020 Proposed Rule Costs: $22.7 billion
TRACKING THE REGULATORY BUDGET
The rulemaking with the highest price tag of the week was the aforementioned DHS proposed rule that focuses on “Employment Authorization for Certain Classes of Aliens With Final Orders of Removal.” The proposal seeks to “eliminate employment authorization eligibility for aliens who have final orders of removal but are temporarily released from custody on an order of supervision with one narrow exception.” The measure’s changes could yield a number of economic effects, including the administrative costs involved in employers and employees certifying their legal status and/or the opportunity costs of lost productivity. DHS estimates that such costs could be roughly $748 million annually, or $5.2 billion in present value. Since it is a proposed rule, however, these costs do not apply to the fiscal year (FY) 2021 regulatory budget. Additionally, given how little time is left in the Trump Administration, its chances at finalization are increasingly slim.
While the administration’s FY 2021 regulatory budget caps are still forthcoming, so far into FY 2021 agencies have officially published 37 deregulatory actions and 10 regulatory actions (as defined by Executive Order (EO) 13,771), totaling $34.4 billion in quantified total net costs. It is worth noting at this point, however, that regardless of whether or not the current administration releases said caps, the Biden Administration assumes power in January 2021. It is highly unlikely that EO 13,771 will remain operative (at least in anything resembling its current form) beyond then, and thus the FY 2021 regulatory budget “window” will be a truncated one. Nevertheless, the American Action Forum (AAF) will continue to track EO 13,771 activity through the end of the administration to provide a record of the regulatory budget initiative’s historical legacy and implications. AAF’s review of the administration’s FY 2020 regulatory budget progress can be found here.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) authorizes the first at-home self-test for COVID-19.
On November 17, the FDA issued an Emergency Use Authorization (EUA) for the first at-home self-testing kit for COVID-19. Previous at-home kits required test samples to be sent to labs to obtain results. The new test can be completed—including result—in about 30 minutes, according to Lucira Health, the test’s creator.
In issuing the EUA, the FDA found that “[b]ased on the totality of scientific evidence available to FDA, it is reasonable to believe that your product may be effective in diagnosing COVID-19, and that the known and potential benefits of your product when used for diagnosing COVID-19, outweigh the known and potential risks of your product.”
The test will be made available to individuals 14 years and older with a doctor’s prescription. In addition to at-home use, it is also authorized for use at the point of care. Lucira Health said in a press release it expects to have the test available nationally by early spring and the cost of the test to run about $50.
According to the FDA and Lucira Health, the sample can be obtained within 2 minutes using a nasal swab. The user then swirls the sample swab in a vial and places the vial in the test unit. A result is indicated in about 30 minutes. The FDA says that those receiving a positive result should self-isolate and consult with a doctor. Those receiving a negative result should follow up with a doctor as “negative results do not preclude an individual from SARS-CoV-2 infection.”
The EUA is the latest good news on advancements in medical science that may help wind down the COVID-19 pandemic. In recent weeks, two potential vaccines have reported 90 percent effectiveness or better in preventing the contraction of the disease. Those vaccines may be granted their own EUAs in the coming weeks once applications are filed.
TOTAL BURDENS
Since January 1, the federal government has published $35.2 billion in total net costs (with $12.5 billion from finalized rules) and 344.5 million hours of net annual paperwork burden increases (with 284.9 million hours due to final rules). Click here for the latest Reg Rodeo findings.