Week in Regulation
April 22, 2019
Tack On $900 Million in The Deregulatory Column
As agencies head into the second half of fiscal year (FY) 2019, last week saw a modest stream of deregulatory measures flow through the pages of the Federal Register. The Department of Health and Human Services (HHS), in particular, promulgated a notable handful of measures – both proposed and final. Between both proposed and final rules last week, agencies published $898.9 million in total net cost savings and cut 110,457 hours of paperwork.
REGULATORY TOPLINES
- New Proposed Rules: 33
- New Final Rules: 66
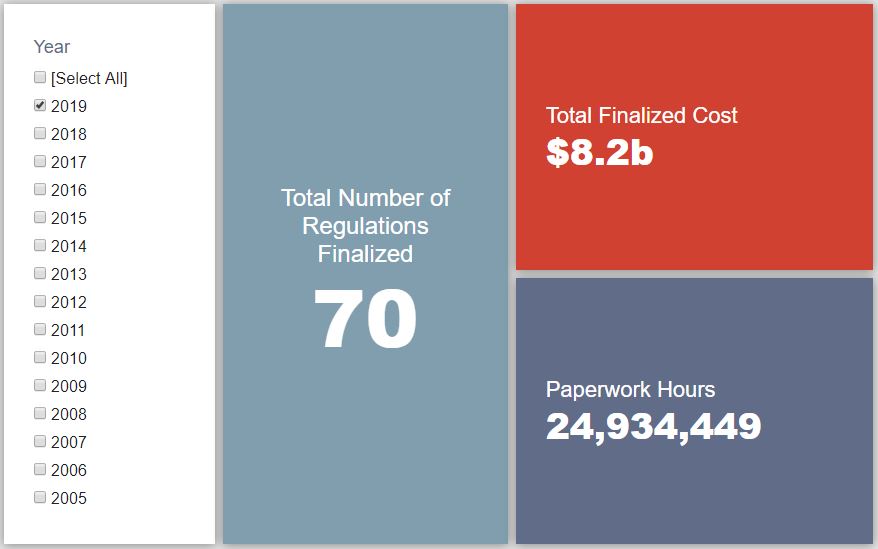
- 2019 Total Pages: 16,564
- 2019 Final Rule Costs: $8.2 Billion
- 2019 Proposed Rule Costs: $5.5 Billion
TRACKING THE REGULATORY BUDGET
The most significant rulemaking with ramifications for the FY 2019 regulatory budget under Executive Order (EO) 13,771 was a final rule out of HHS updating a bevy of programs related to Medicare and Medicaid. Many of these changes come from statutory directives from the Bipartisan Budget Act of 2018. Through such actions as increasing the availability of telehealth options and streamlining redundant reporting requirements, HHS expects to produce roughly $534 million in net cost savings from 2020 through 2029.
Another notable deregulatory action from HHS comes out of its sub-agency, the Administration for Children and Families, that addresses the Adoption and Foster Care Analysis and Reporting System (AFCARS) program. This proposed rule seeks “to streamline the AFCARS data elements that were finalized in the AFCARS final rule published on December 14, 2016.” The agency estimates that such changes could reduce annual paperwork by 544,337 hours, bringing $39.2 million in commensurate savings. Interestingly, the proposal makes a point of noting how this measure came as a result of HHS’s “Regulatory Reform Task Force” established under EO 13,777.
So far in FY 2019 (which began on October 1, 2018), there have been 43 deregulatory actions (per the rubric created by EO 13,771 and the administration’s subsequent guidance document) against 18 rules that increase costs and fall under the EO’s reach. Combined, these actions yield quantified net costs of roughly $9.3 billion. This total, however, includes the caveat regarding the baseline in the Department of Agriculture’s “National Bioengineered Food Disclosure Standard.” If one considers that rule to be deregulatory, the administration-wide net total is approximately $2.6 billion in net costs. The administration’s cumulative savings goal for FY 2019 is approximately $18 billion.
THIS WEEK’S REGULATORY PICTURE
One can describe “regulatory policy” in many ways: mundane, opaque, monotonous, complex, legalistic. The list goes on. In order to help provide a clearer and more straight-forward view into this world, the American Action Forum (AAF) will seek to provide a brief illustration of a notable regulatory trend we have identified in a given week. This week’s entry: The Food and Drug Administration’s sunscreen rule strikes again.
Seven weeks ago, AAF wrote about the long road for an FDA proposed rule on sunscreen ingredients that can be use in over-the-counter products. That road got just a little bit longer this week.
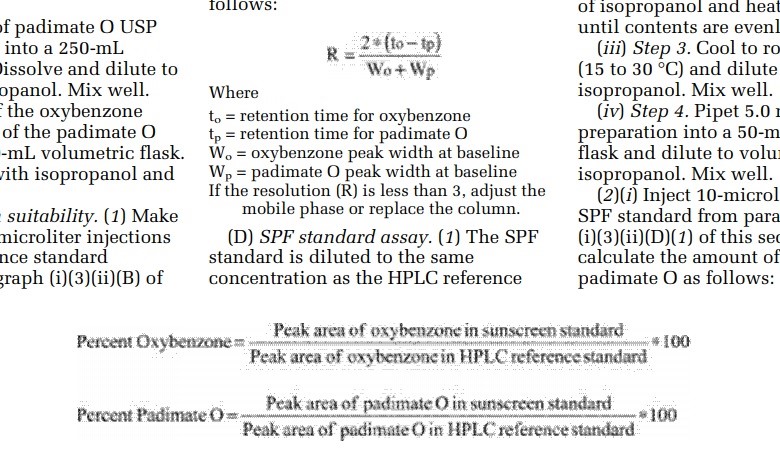
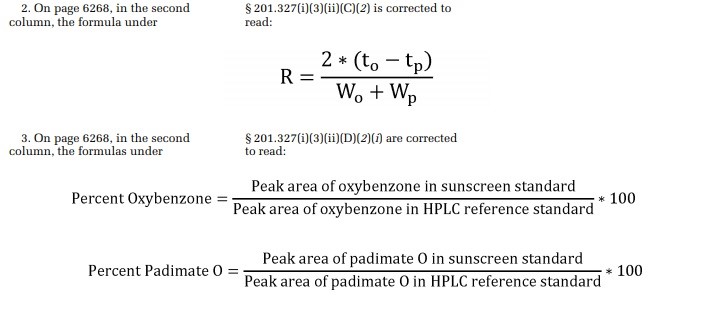
The FDA published a correction to the February 26 proposed rule this week because the formulas included in that notice were illegible. Indeed, as the top image above shows, the graininess of the formulas could prove tricky for some to read. The bottom image shows the formulas in their larger, clearer corrected format.
Agencies frequently publish corrections to previous Federal Register documents. Between April 1 and April 19, 22 such notices have appeared. Typically, however, corrections stem from typographical errors, incorrect data in tables and charts, and missing words that could cause confusion. This correction stands out in that the information is correct, albeit not the easiest to read.
The same day that FDA published its correction, it also published a notice extending the public comment period. It did not, however, cite the correction as a reason for the delay. Despite the comment period extension, the FDA wrote it does not believe the 30-day extension will significantly delay the rulemaking.
TOTAL BURDENS
Since January 1, the federal government has published $13.7 billion in net costs (with $8.2 billion in finalized costs) and 25.1 million hours of net paperwork burden increases (including roughly 24.9 million hours from final rules). Click here for the latest Reg Rodeo findings.