Week in Regulation
April 29, 2019
Modest Actions Add Up to Balanced Week
Last week, there were 14 rulemakings that provided some sort of quantified cost or savings estimate – a fairly busy week in that regard. These actions, however, did not amount to a significant change in 2019’s overall regulatory tally. No regulation had an economic impact in excess of $60 million. Between both proposed and final rules last week, agencies published $12.8 million in total net costs and cut 71,704 hours of paperwork.
REGULATORY TOPLINES
- New Proposed Rules: 41
- New Final Rules: 45
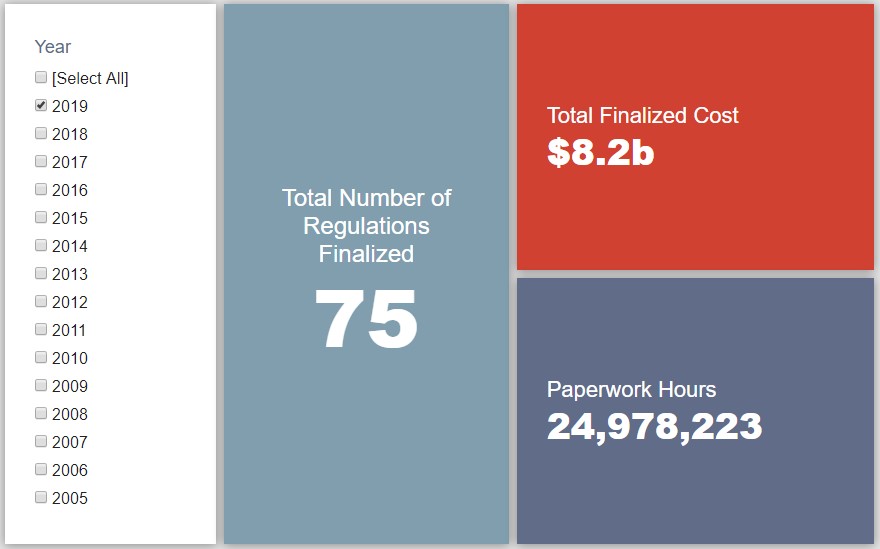
- 2019 Total Pages: 17,892
- 2019 Final Rule Costs: $8.2 Billion
- 2019 Proposed Rule Costs: $5.5 Billion
TRACKING THE REGULATORY BUDGET
The only action that applies to the fiscal year (FY) 2019 regulatory budget established by Executive Order (EO) 13,771 was a deregulatory action from the Department of Health & Human Services (HHS). The HHS rule sets out the “Benefit and Payment Parameters for 2020,” as required annually under the Affordable Care Act. HHS took this year’s iteration of the regulatory program to include certain reforms seeking to increase flexibility for stakeholders. Per agency estimates, this could yield roughly $58 million in cost reductions over 5 years.
The second-most deregulatory action – albeit simply a proposed rule – of the week comes from the Environmental Protection Agency (EPA). The EPA proposal seeks to adjust “Chemical Data Reporting requirements” under the “Toxic Substances Control Act” in a way that further relieves the reporting burden of affected entities. The agency estimates that this could yield more than $18 million in cost savings over 10 years. These savings may actually hit the FY 2020 regulatory budget’s balance sheet as the administration currently expects it to become final in October 2019
So far in FY 2019 (which began on October 1, 2018), there have been 44 deregulatory actions (per the rubric created by EO 13,771 and the administration’s subsequent guidance document) against 18 rules that increase costs and fall under the EO’s reach. Combined, these actions yield quantified net costs of roughly $9.2 billion. This total, however, includes the caveat regarding the baseline in the Department of Agriculture’s “National Bioengineered Food Disclosure Standard.” If one considers that rule to be deregulatory, the administration-wide net total is approximately $2.5 billion in net costs. The administration’s cumulative savings goal for FY 2019 is approximately $18 billion.
THIS WEEK’S REGULATORY PICTURE
One can describe “regulatory policy” in many ways: mundane, opaque, monotonous, complex, legalistic. The list goes on. In order to help provide a clearer and more straight-forward view into this world, the American Action Forum (AAF) will seek to provide a brief illustration of a notable regulatory trend we have identified in a given week. This week’s entry: The Office of Management and Budget’s (OMB) memorandum on “Improving Implementation of the Information Quality Act” (IQA).

For the second time in 13 days, OMB Acting Director Russell T. Vought issued a memo to agency heads with implications on the information used to formulate regulatory decisions. An April 11 memo set forth a more formal process to help OMB comply with the Congressional Review Act. The latest memo, issued on April 24, calls on agencies to improve their processes for complying with the IQA.
The IQA requires OMB to set government-wide guidelines to ensure the data agencies disseminate and rely on for regulatory decisions meet certain standards, including making data publicly available and subject to correction, if necessary. Those guidelines were issued in 2002. The new memo aims to improve agency compliance with the IQA guidelines by incorporating best practices and establishing clearer timelines for public responses.
An important part of the memo specifies that “complex models” used by agencies to inform the development of economically significant regulations should be peer reviewed before those regulations are drafted and submitted to the Office of Information and Regulatory Affairs. While no consequences are specified in the memo, this could have the effect of adding more time to the front end of the rulemaking process. Ostensibly, however, agencies were already expected to meet this objective – so if rules are now delayed it will be because the expectation is now being enforced.
Another component of the memo with ramifications for agencies has to do with responding to the public’s requests for corrections. The memo explains that agencies have struggled, or outright failed, to respond to such requests in a timely manner. To help solve this problem, OMB expects agencies to revise their internal procedures to respond to these inquiries within 120 days.
TOTAL BURDENS
Since January 1, the federal government has published $13.7 billion in net costs (with $8.2 billion in finalized costs) and 25 million hours of net paperwork burden increases (almost entirely from final rules). Click here for the latest Reg Rodeo findings.