Week in Regulation
May 28, 2019
A Pair of Health Care Rules Add Up to Net Regulatory Week
Last week, the administration released its spring Unified Agenda laying out its regulatory and deregulatory plans for the coming year. In the pages of the Federal Register, though, it turned out to be more on the regulatory side. Two rules out of the Department of Health and Human Services (HHS) led the charge. Between both proposed and final rules last week, agencies published $1.5 billion in total net costs and added 1.8 million hours of paperwork.
REGULATORY TOPLINES
- New Proposed Rules: 46
- New Final Rules: 77
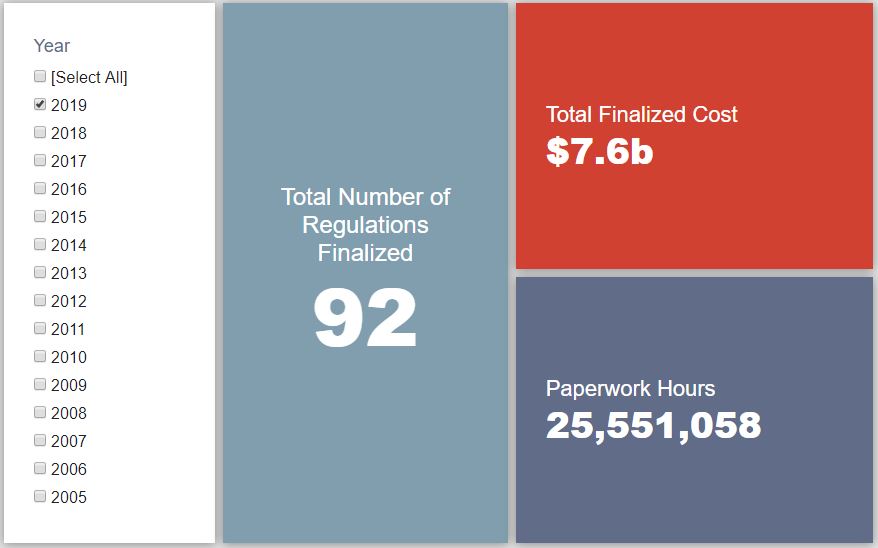
- 2019 Total Pages: 24,315
- 2019 Final Rule Costs: $7.6 Billion
- 2019 Proposed Rule Costs: $5.4 Billion
TRACKING THE REGULATORY BUDGET
The most significant HHS action of the week was the final rule regarding “Protecting Statutory Conscience Rights in Health Care; Delegations of Authority.” The rule reasserts the authority of the Office for Civil Rights to monitor and scrutinize “recipients of HHS funds” to ensure they “comply with their legal obligations” regarding certain freedom of conscience laws. This comes largely as a response to changes the Obama Administration made in this same space. Although the primary debate surrounding these actions centers around deeply contentious social and moral issues, the new regulatory framework established under this rule has economic costs as well. HHS estimates that the various compliance requirements could cost affected entities roughly $900 million over five years.
The other regulatory action from HHS with implications for the fiscal year (FY) 2019 regulatory budget under Executive Order (EO) 13,771 is this rule entitled “Modernizing Part D and Medicare Advantage To Lower Drug Prices and Reduce Out-of-Pocket Expenses.” The rule seeks to increase “negotiation leverage for MA and Part D plans” in order to help drive down the cost of prescription drugs. HHS estimates that the compliance requirements established under this rule could yield $217 million in total costs. Between these two rules, HHS now has $1.1 billion more in costs under the “regulatory” column of its FY 2019 regulatory budget.
So far in FY 2019 (which began on October 1, 2018), there have been 48 deregulatory actions (per the rubric created by EO 13,771 and the administration’s subsequent guidance document) against 22 rules that increase costs and fall under the EO’s reach. Combined, these actions yield quantified net costs of roughly $8.6 billion. This total, however, includes the caveat regarding the baseline in the Department of Agriculture’s “National Bioengineered Food Disclosure Standard.” If one considers that rule to be deregulatory, the administration-wide net total is approximately $1.9 billion in net costs. The administration’s cumulative savings goal for FY 2019 is approximately $18 billion.
THIS WEEK’S REGULATORY PICTURE
One can describe “regulatory policy” in many ways: mundane, opaque, monotonous, complex, legalistic. The list goes on. In order to help provide a clearer and more straight-forward view into this world, the American Action Forum will seek to provide a brief illustration of a notable regulatory trend we have identified in a given week. This week’s entry: one of the most granular cost estimates we’ve come across.

This clip comes from the Medicare rule discussed above. One of the requirements under the rule is for affected plan sponsors to include further information on changes in drug prices in their “Explanation of Benefits” (EOB). HHS estimates that this should only take roughly one additional page. No big deal, right? Actually, it starts to add up to the tune of nearly $6 million in the aggregate.
Most cost estimates focus on such things as labor costs or significant capital investments. This one drills down into the cost of a single sheet of paper with words printed on it. The paper costs half a cent. The toner that puts words on that paper costs half a cent. And the additional postage required due to the additional weight comes out to 38 ten-thousandths of a cent. Since there are apparently 47.6 million of these mailings, that all comes out to a total cost of $5.7 million. That kind of business ought to please the “people person’s paper people.”
TOTAL BURDENS
Since January 1, the federal government has published $13 billion in net costs (with $7.6 billion in finalized costs) and 27.3 million hours of net paperwork burden increases (with 25.6 million coming from final rules). Click here for the latest Reg Rodeo findings.