Week in Regulation
June 27, 2022
Proposed Pool Rule Rings in Summer
As spring crossed into summer this week, a proposed rule regarding pool pumps was a timely highlight among rules published in the Federal Register. Meanwhile, regulatory policy enthusiasts celebrated the release of the latest regulatory agenda. Across all rulemakings, agencies published $1.8 billion in total net costs and added 14.6 million annual paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 34
- Final Rules: 33
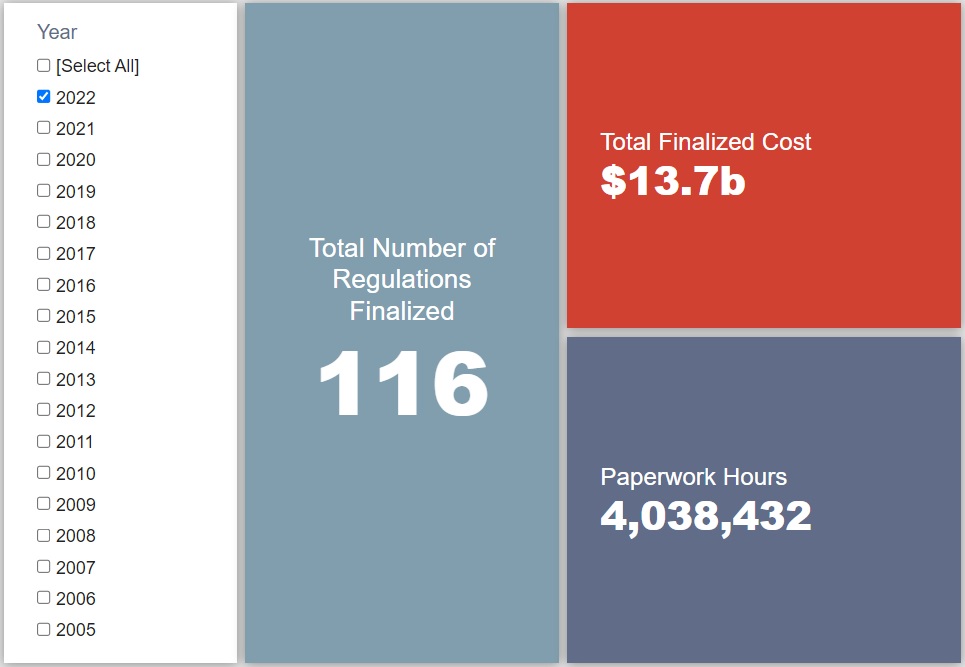
- 2022 Total Pages: 37,918
- 2022 Final Rule Costs: $13.7 billion
- 2022 Proposed Rule Costs: $78.1 billion
NOTABLE REGULATORY ACTIONS
The Department of Energy’s (DOE) latest energy efficiency proposed rule stood out this past week. Appropriate for summer, the rule would establish energy conservation standards for pool pump motors. DOE estimates the lifetime cost of the proposal will be $1.5 billion.
Another notable proposal emerged from the Department of Health and Human Services, which published its calendar year 2023 update to Medicare’s home health service payment rates. The proposed rule includes updates to affiliated reporting programs that would cost $267 million by adding 14.6 million hours of paperwork annually.
A lone final rule contained quantified estimates this week, as a Federal Communications Commission rule on 911 Fee Diversion added 3,630 hours of annual paperwork.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the current administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
This week from the Biden Administration was a snoozer. As mentioned above, it published just one final rule with a quantified estimate, adding a mere 3,630 annual hours of paperwork. The Trump Administration trimmed regulatory costs by about $264 million thanks to a rule by the Securities and Exchange Commission that allowed investment companies to make certain required informational documents available electronically rather than having to send them through the mail. In contrast, the Obama Administration added nearly $1 billion through an Environmental Protection Agency rule that updated air quality standards.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) makes headlines with actions on nicotine products.
On June 21, the Office of Information and Regulatory Affairs, which coordinates and reviews rulemaking activities of federal agencies, released its “Spring 2022 Unified Agenda of Federal Regulatory and Deregulatory Actions.” Better known as the regulatory agenda, the plan is published twice per year and includes the rules agencies plan to work on over the next 12 months (but not necessarily complete).
In the latest edition, the FDA stole the show by including an entry for a new rule that would set a maximum nicotine level in cigarettes. FDA says the rule would reduce the addictiveness of cigarettes, “thus giving addicted users a greater ability to quit.” It also believes such a standard would help prevent older kids and young adults from becoming regular smokers.
The FDA cites statutory authority that allows the agency to set tobacco product standards and includes specific authorization for standards “appropriate for the protection of the public health” including for “nicotine yields of the product.” The agency gave no estimated timeline for finalizing the rule and does not plan to publish a proposed rule until May of 2023.
The revelation of the rulemaking was just one headline-grabbing action from the FDA related to nicotine this week; on June 23, the agency announced it was ordering all electronic cigarettes from manufacturer JUUL Labs off the market. The agency says it made the decision based on its determination “that the applications lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
TOTAL BURDENS
Since January 1, the federal government has published $91.8 billion in total net costs (with $13.7 billion in new costs from finalized rules) and 65.5 million hours of net annual paperwork burden increases (with 4 million hours in increases from final rules).