Week in Regulation
December 19, 2025
Another Light Week, but with Some Executive Orders
Much like the week before it, this past week of regulatory activity did not see much in the way of major rulemakings – at least in terms of overall economic impact. If anything, the week’s main news may have been a series of executive orders (EOs) that could eventually have major ramifications. There were seven rulemakings with some kind of measurable impact. The most significant of these was a Department of Health & Human Services (HHS) proposal that would restrict hospitals receiving Medicare and Medicaid funding from providing gender reassignment procedures for minors. Across all rulemakings, federal agencies published roughly $45.9 million in total costs and added 46,538 paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 35
- Final Rules: 56
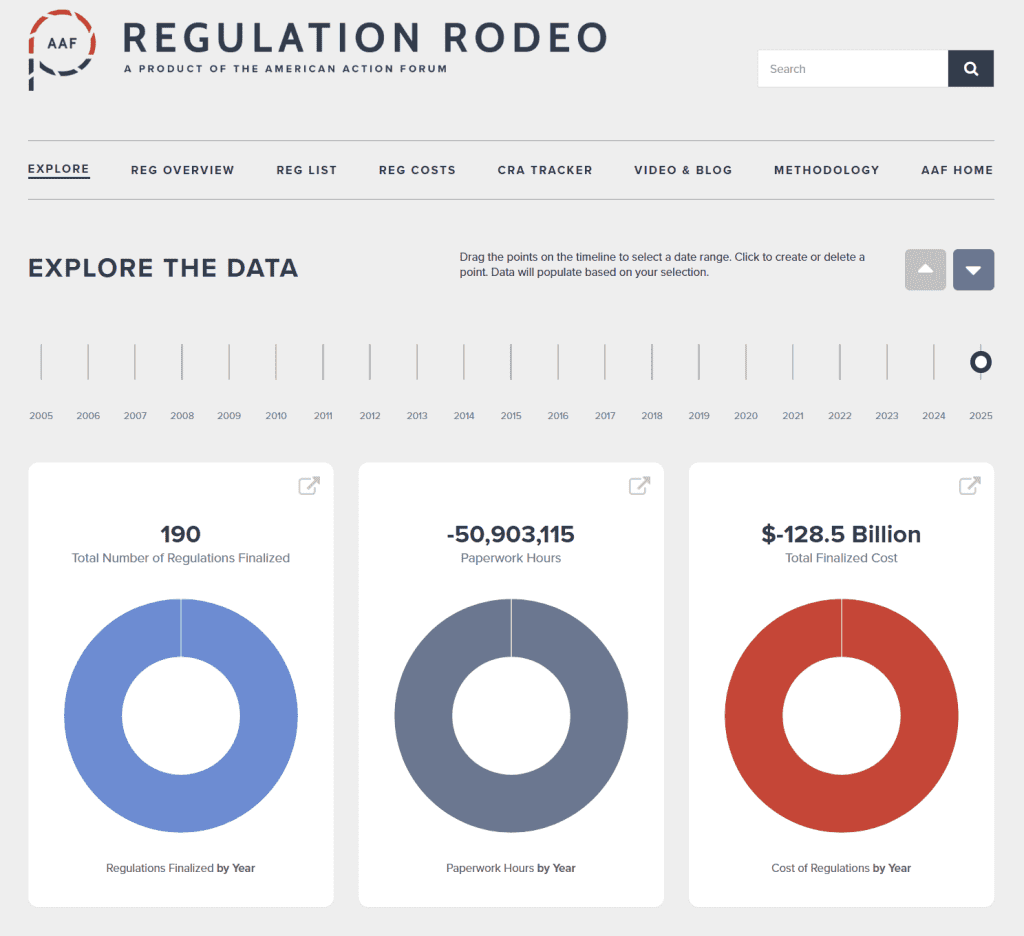
- 2025 Total Pages: 59,595
- 2025 Final Rule Costs: -$128.5 billion
- 2025 Proposed Rule Costs: -$847.5 billion
NOTABLE REGULATORY ACTIONS
As mentioned above, the most consequential rulemaking of the week was the proposed rule from HHS titled “Medicare and Medicaid Programs; Hospital Condition of Participation: Prohibiting Sex-Rejecting Procedures for Children.” As the title suggests, the agency seeks to leverage its authority under the Social Security Act to “prohibit Medicare-participating hospitals” from conducting a series of relevant procedures. The rulemaking came as part of a set of related proposed rules seeking to restrict gender-related procedures more broadly. Outside of the provocative social and cultural issues at hand, this rulemaking in particular would bring an estimated $51.3 million in total costs between the cost for affected patients to find alternative care providers and for the administrative costs to affected entities in updating their operations.
TRACKING TRUMP 2.0
The main regulatory news of the week from the White House was a trio of EOs issued by President Trump. In a bit of a whiplash-inducing development, the president started the week by designating one drug as a Weapon of Mass Destruction (WMD) and then finished it by taking initial steps to loosen the legal restraints on another drug and establishing an overarching space policy. The first EO that designates “illicit fentanyl” as a WMD primarily concerns itself with certain national security issues, but the designation would seem to imply regulatory implications for the international transit of such things as the “core precursor chemicals” it also covers. The EO on “Increasing Medical Marijuana and Cannabidiol Research” initiates the “process related to rescheduling marijuana to Schedule III,” which would remove the strict constraints it currently faces due to its “Schedule I” classification under the Controlled Substances Act. Finally, the “Ensuring American Space Superiority” EO sets some nominal space travel-related goals, but also directs relevant agencies to review and update regulatory provisions regarding the development, manufacturing, and deployment of space-related technologies.
There was a blip of Congressional Review Act (CRA) news before lawmakers dispersed for the holiday recess. The Senate held a vote on S.J. Res 82, a resolution seeking to repeal a Trump Administration HHS rulemaking. The vote failed on a 50-50 margin.
The AAF CRA tracker provides a full survey of activity under the law thus far in 2025. As of today, members of the 119th Congress have introduced CRA resolutions of disapproval addressing 70 rulemakings across the Biden and Trump Administrations that collectively involve $138 billion in compliance costs. Of these, 22 have been passed into law[1], repealing a series of Biden Administration rules that had a combined $3 billion in associated compliance costs – roughly 2 percent of that potential $138 billion total. While the main window of CRA action has largely passed, there are still outstanding resolutions that could move legislatively. AAF will continue to monitor and update such developments as appropriate.
TOTAL BURDENS
Since January 1, the federal government has published $976 billion in total regulatory net cost savings (with $128.5 billion in cost savings from finalized rules) and 75.1 million hours of net annual paperwork cuts (with 50.9 million hours coming from final rules).