Week in Regulation
August 14, 2023
Biden Administration Goes Past $400 Billion in Final Rule Costs
Last week saw a steady stream of regulatory activity with notable impacts and a variety of subject matter. All told, there were 14 rulemakings with some quantifiable economic impact. The most significant rulemakings covered such items as accessibility guidelines for pedestrian infrastructure including sidewalks and changes to how various entities report air emissions data to the Environmental Protection Agency (EPA). Across all rulemakings, agencies published $10 billion in total costs and added 3 million annual paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 39
- Final Rules: 70
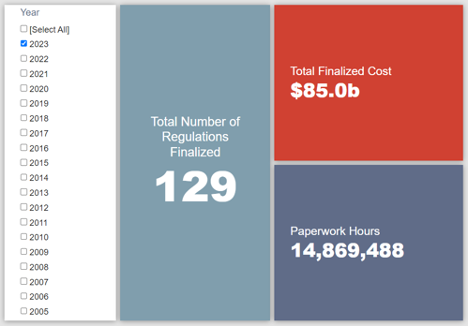
- 2023 Total Pages: 54,783
- 2023 Final Rule Costs: $85 billion
- 2023 Proposed Rule Costs: $271.5 billion
NOTABLE REGULATORY ACTIONS
The most consequential rulemaking of the week was the final rule from the Architectural and Transportation Barriers Compliance Board (ATBCB) regarding “Accessibility Guidelines for Pedestrian Facilities in the Public Right-of-Way.” The rule institutes new standards to “ensure that sidewalks, pedestrian street crossings, pedestrian signals, and other facilities for pedestrian circulation and use constructed or altered in the public right-of-way by state and local governments are readily accessible to and usable by pedestrians with disabilities.” The rule has had a long and winding regulatory history, with its original proposed rule iteration coming all the way back in 2011. ATBCB primarily sets its estimates in annualized terms given the variety of timeframes – varying from 15 to 50 years – involved with the types of facilities included and expects the implementation costs to be $196.7 million per year. Extending the specific annualized cost components across their respective timelines (as listed by ATBCB) adds up to nearly $4.4 billion.
The other notable rulemaking of the week was the proposed rule from EPA regarding “Revisions to the Air Emissions Reporting Requirements.” In particular, the proposal seeks “to require certain sources report information regarding emission of hazardous air pollutants (HAP); certain sources to report criteria air pollutants, their precursors and HAP; and to require State, local, and certain tribal air agencies to report prescribed fire data.” The rulemaking would have a staggered effect. As EPA notes: “the proposed rule’s total cost impact is estimated at $117.4 million on average annually from 2024 to 2026, and then is estimated at $477.9 million in 2027.” Extending that dynamic across a 10-year window, the agency finds that total present value costs would add up to roughly $2.4 billion.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the current administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
With the accessibility guidelines rule discussed above, the Biden Administration’s final rule cost total now exceeds $400 billion. For context, it would take the Obama Administration an additional 14 months to go past that threshold in October 2012. For the beginning of August 2011, however, there was a $2.7 billion uptick in costs for the Obama total. A Department of Homeland Security rule on “Air Cargo Screening” provided the bulk of that shift. There was little to report on regarding the Trump Administration’s regulatory activity circa early August 2019, though.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) takes another look at its regulatory standards for “trans fats” in such items as canned tuna and peanut butter.
Source: Photo by personalgraphic.com on Unsplash
Last Wednesday, FDA published a direct final rule (and an accompanying proposed rule) regarding “Revocation of Uses of Partially Hydrogenated Oils in Foods.” The rulemaking builds upon efforts from a 2015 FDA order that found “there is no longer a consensus among qualified experts that PHOs [partially hydrogenated oils], the primary dietary source of industrially produced trans fatty acids (IP–TFA), are GRAS [‘generally recognized as safe’] for any use in human food.” While that order required either the removal of certain items from the market or changes in their ingredients, some exceptions carried on. This rulemaking now seeks to address some of those exceptions.
In particular, the rule would: “remove PHOs as an optional ingredient in the standards of identity for peanut butter and canned tuna, and remove partially hydrogenated menhaden oil, fish oil, and rapeseed oil from FDA’s regulations affirming food substances as GRAS.” Additionally, the rule revokes the “prior-sanctioned uses of PHOs in margarine, shortening, and bread, rolls, and buns.” The “prior-sanctioned use” provision essentially grandfathered-in any items that FDA generally “sanctioned or approved prior to September 6, 1958.”
While these changes, particularly those involving optional ingredients, may seem rather mundane and inconsequential, there will be some nominal impact to entities that produce the relevant items. Covered entities may now need to change the basic formulation of their product and update the labels for such products. Additionally, FDA expects there to be some degree of economic disruption for these entities in instances where the reformulation of their product results in changes to consumer demand. The agency estimates that the costs involved with this will add up to $260 million in total over a 20-year horizon.
FDA is issuing this rulemaking as “direct final rule” noting that “this rule is appropriate for direct final rulemaking because it includes only noncontroversial amendments, and we anticipate no significant adverse comments.” In case there are adverse comments, however, it has also produced a substantively similar proposed rule as a companion rulemaking. Interested parties have until October 23 to submit comments.
TOTAL BURDENS
Since January 1, the federal government has published $356.5 billion in total net costs (with $85 billion in new costs from finalized rules) and 165.6 million hours of net annual paperwork burden increases (with 14.9 million hours in increases from final rules).