Week in Regulation
February 28, 2022
Cross-Cutting Billion-Dollar Proposals Highlight Short Week
Despite the holiday-shortened length, this past week produced one of the more active regulatory hauls in recent months. A pair of proposed rules – from the Food & Drug Administration (FDA) and Securities & Exchange Commission (SEC), respectively – brought economic impacts measured in the billions of dollars. These proposals, however, came from opposite sides of the deregulatory/regulatory axis, thus producing a mixed week overall. Across all rulemakings, agencies published $1.6 billion in total net cost savings but added 2.9 million annual paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 37
- Final Rules: 44
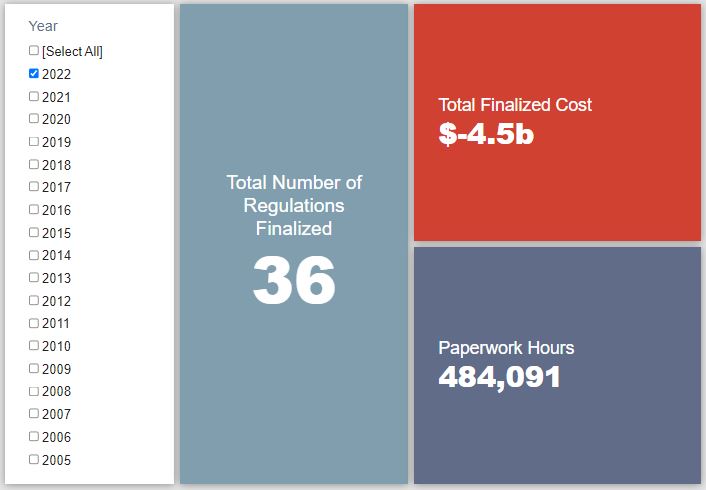
- 2022 Total Pages: 10,907
- 2022 Final Rule Costs: -$4.5 billion
- 2022 Proposed Rule Costs: -$1.2 billion
NOTABLE REGULATORY ACTIONS
The most significant rulemaking of the week was an FDA proposed rule regarding “Medical Devices; Quality System Regulation Amendments.” The proposal seeks to update medical device manufacturing standards to “align more closely with the international consensus standard for devices by converging with the quality management system (QMS) requirements used by other regulatory authorities from other jurisdictions (i.e., other countries).” FDA expects that removing the need to follow two separate, redundant sets of standards will reduce costs for affected manufacturers. Per the rulemaking’s analysis, these cost savings net out to roughly $526 million annually (or nearly $5.3 billion over a 10-year period) with 1.9 million hours in associated paperwork reductions.
On the other side of the ledger, however, comes an SEC proposal regarding “Shortening the Securities Transaction Settlement Cycle.” As the rulemaking’s title suggests, SEC is proposing to “shorten the standard settlement cycle for most broker-dealer transactions from two business days after the trade date (‘T+2’) to one business day after the trade date (‘T+1’).” The agency believes this action will help “to protect investors, reduce risk, and increase operational efficiency.” As one can expect, however, updating these transaction settlement systems will involve some costs. SEC currently estimates total, industry-wide costs of approximately $3.5 billion with an additional 2.8 million hours of paperwork annually due to new compliance requirements.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the new administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the American Action Forum (AAF) RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
![]()
For yet another week, the final rules coming out of the Biden Administration had a limited impact. As such, the current administration’s to-date tallies remained fairly stagnant. The major moves across the three covered administrations came from the Trump-era cost total and the Obama-era paperwork total. A Trump Administration FDA rule regarding medical device tests brought $232 million in increased costs. For the Obama-era shift, there was a nearly 900,000-hour increase, with a Federal Reserve “Truth in Lending” rule providing the majority of that spike.
THIS WEEK’S REGULATORY PICTURE
This week, newly proposed animal welfare standards for birds.

Source: “Rainbow Lorikeet parrot” by David Clode
For those interested in bird law, the U.S. Department of Agriculture (USDA) published a notice in the February 22 edition of the Federal Register that it is proposing to establish “Standards for Birds Not Bred for Use in Research Under the Animal Welfare Act (AWA).”
The AWA was enacted in 1966 to set requirements for the “humane handling, care, treatment, and transportation of certain animals by dealers, research facilities, exhibitors, operators of auction sales, and carriers and intermediate handlers.” The 2002 Farm Bill included language that expanded the definition of an “animal” under the act and was written in such a way that birds not used in research could, for the first time, get coverage under the USDA’s regulations.
The proposed rule published this week is a victory for those who have pushed the USDA for nearly 20 years to set standards for birds in captivity. In 2004, USDA published an advance notice of proposed rulemaking seeking feedback from the public on how to best set standards for birds, rats, and mice not used in research. When nothing had emerged nine years later, several groups sued the agency for failing to act.
In 2020, a federal appeals court finally ruled that USDA was required to establish standards. The agency conducted listening sessions and received more than 10,000 written public comments to form the basis of the proposed rule. Under the court order, USDA is to issue a final rule within one year of this week’s proposal getting published.
TOTAL BURDENS
Since January 1, the federal government has published $5.7 billion in total net cost savings (with $4.5 billion in new cost savings from finalized rules) and 6.2 million hours of net annual paperwork burden increases (with 484,091 hours in increases from final rules).