Week in Regulation
May 23, 2022
Energy Efficiency Trend Continues
A trend is developing. In the week before last, the main regulatory items involved Department of Energy (DOE) energy efficiency standards for “general service lamps.” This past week, updated DOE standards for “commercial water heating equipment” stepped into the limelight, albeit at a much lower magnitude than the preceding week’s developments. Across all rulemakings, agencies published $781.1 million in total net costs and added 64,699 annual paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 28
- Final Rules: 61
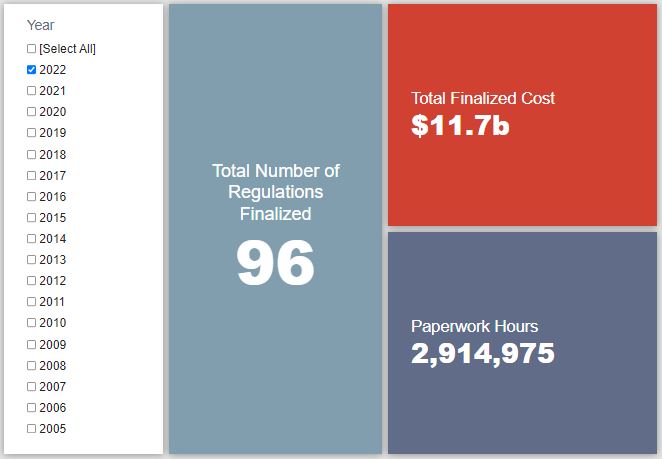
- 2022 Total Pages: 31,045
- 2022 Final Rule Costs: $11.7 billion
- 2022 Proposed Rule Costs: $75 billion
NOTABLE REGULATORY ACTIONS
The most significant rulemaking of the week was DOE’s proposed rule regarding “Energy Conservation Program: Energy Conservation Standards for Commercial Water Heating Equipment.” The proposal is yet another iteration of DOE seeking to update efficiency standards for various consumer products. In this instance, the agency addresses the regulations involving “certain commercial and industrial equipment, including commercial water heaters, hot water supply boilers, and unfired hot water storage tanks.” Per the rulemaking’s analysis, these updated standards would bring $600 million in total “Incremental Product Costs,” or $59.2 million on an annual basis.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the current administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
![]()
Since the week’s main action came on the proposed rule side, there was limited movement in the Biden Administration’s final rule regulatory tallies. In fact, it was a relatively muted week for each of the three administrations covered here. The Obama Administration saw the most movement, with nearly $104 million in new costs and more than 100,000 hours of paperwork added. These increases came from a handful of rules, but the Department of Labor – in separate rules – provided the bulk of both costs and paperwork changes.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) takes a step to address the infant formula shortage.
On May 16, the FDA issued guidance to manufacturers that it would consider exercising “enforcement discretion” for some of the agency’s infant formula requirements. The guidance is intended to increase imports of formula in response to a nationwide shortage, exacerbated by a February safety recall of product by Abbott Nutrition, the largest formula manufacturer in the United States.
The enforcement discretion, to be considered on a case-by-case basis, applies to certain labeling and nutrient requirements. FDA asked manufacturers that do not currently make formula that complies with FDA requirements – primarily domestic manufacturers that only make formula for export and manufacturers with foreign facilities that do not export formula to the United States – to provide documentation to the agency to potentially waive some of the requirements. These waivers would allow formulas that typically fail to comply with FDA regulations to be sold in the United States.
The documentation for individual products includes labeling descriptions, batch test results, and the name of the countries where the formula is currently sold. Each manufacturing facility must provide a certification of good manufacturing processes and its FDA Food Facility Registration number, among other documentation. The FDA guidance will remain in effect until at least November 22, 2022.
The guidance is a component of a Biden Administration plan to address the infant formula shortage. On May 18, President Biden unveiled a second part of the plan by signing a memorandum delegating authority to the Department of Health and Human Services under the Defense Production Act to address the shortage.
The shortage highlights the unintended consequences that can stem from regulatory requirements. In this case, the structure of FDA’s requirements (along with broader protectionist trade and other poorly designed policies) led to a situation where one manufacturer produced the bulk of a needed good. When that manufacturer faltered, the lack of a competitive environment left an insufficient supply of alternatives.
TOTAL BURDENS
Since January 1, the federal government has published $86.7 billion in total net costs (with $11.7 billion in new costs from finalized rules) and 48.5 million hours of net annual paperwork burden increases (with 2.9 million hours in increases from final rules).