Week in Regulation
March 4, 2019
Sunscreen Dominates Last Week of February
The final week of February 2019 brought yet another regulatory week. A single proposed rule regarding over-the-counter (OTC) sunscreen standards out of the Food & Drug Administration (FDA) provided the bulk of new costs in what would have otherwise been a fairly mundane week. This proposal also provided an interesting example of how long and winding the regulatory road can get. Between both proposed and final rules last week, agencies published $646.9 million in total net costs, as well as 905,994 hours of new paperwork.
REGULATORY TOPLINES
- New Proposed Rules: 44
- New Final Rules: 51
- 2019 Total Pages: 7,238
- 2019 Final Rule Costs: $8.9 Billion
- 2019 Proposed Rule Costs: $3.5 Billion
TRACKING THE REGULATORY BUDGET
The FDA sunscreen proposal was the clearly the preeminent rulemaking of the week. The proposal seeks to “update and make effective regulations to ensure the safety and effectiveness of sunscreen products marketed under the OTC drug monograph.” These amended standards could bring total, undiscounted costs of nearly $620 million over 20 years. Since this is still only a proposed rule, it does not yet count as a regulatory action for the purposes fiscal year (FY) 2019’s regulatory budget under Executive Order (EO) 13,771.
So far in FY 2019 (which began on October 1, 2018), there have been 31 deregulatory actions (per the rubric created by EO 13,771 and the administration’s subsequent guidance document) against 12 rules that increase costs and fall under the EO’s reach. Combined, these actions yield quantified net costs of roughly $10.2 billion. This total, however, includes the caveat regarding the baseline in the Department of Agriculture’s “National Bioengineered Food Disclosure Standard.” If one considers that rule to actually be deregulatory, the administration-wide net total is approximately $3.5 billion in net costs. The administration’s cumulative savings goal for FY 2019 is approximately $18 billion.
THIS WEEK’S REGULATORY PICTURE
One can describe “regulatory policy” in many ways: mundane, opaque, monotonous, complex, legalistic. The list goes on. In order to help provide a clearer and more straight-forward view into this world, the American Action Forum will seek to provide a brief illustration of a notable regulatory trend we have identified in a given week. This week’s entry: Rulemaking is Hard – Sunscreen Edition.
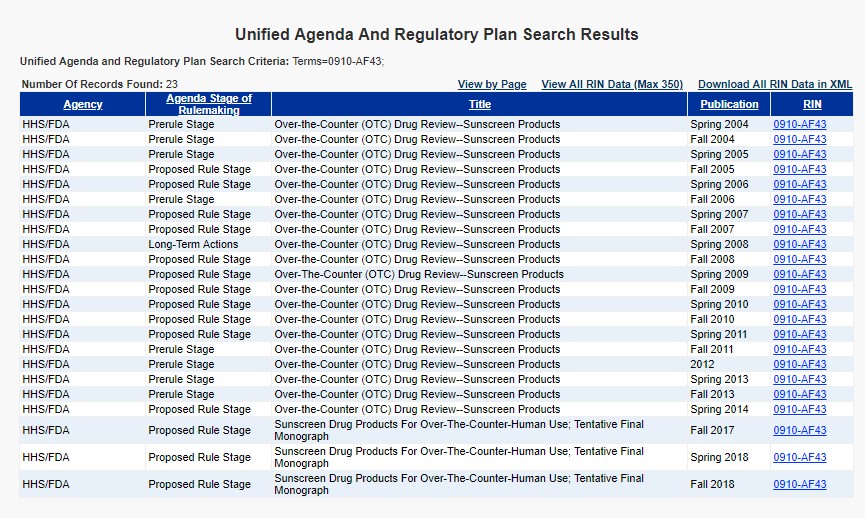
The image above illustrates the lengthy odyssey of the proposed sunscreen standards. The picture shows that this regulation (per the consistent “Regulation Identifier Number” or RIN) has officially been under consideration for roughly 15 years. The full story of the rulemaking goes back even further. In 1999, the FDA published a final rule identifying 16 sunscreen ingredients with “general recognition of safety and effective” status for over-the-counter (OTC) use. Before the rule ever went into effect, however, the FDA stayed the rule indefinitely in 2001 to “provide additional time to resolve various outstanding issues, such as the labeling and testing of finished OTC sunscreen products.” This proposal is the latest effort to move the regulatory ball forward, but it is still not the end of the road. There is, however, light at the end of the tunnel as the Sunscreen Innovation Act of 2014 directs FDA to produce a final rule on the topic by November 26, 2019.
TOTAL BURDENS
Since January 1, the federal government has published $12.4 billion in net costs (with $8.9 billion in finalized costs) and 19.3 million hours of net paperwork burden increases (including roughly 24.1 million hours from final rules). Click here for the latest Reg Rodeo findings.












