Weekly Checkup
July 1, 2022
FDA Proposes Allowing Some Prescription-Only Drugs to be OTC
With everything going on, you may have missed a new announcement from the Food and Drug Administration (FDA). The agency has released a notice for proposed rulemaking, entitled “Nonprescription Drug Product with an Additional Condition for Nonprescription Use (ACNU),” that would allow certain prescription drugs to be purchased without a prescription, provided the manufacturer provides additional labeling and other instructions for use, and enforces certain conditions for the patient to meet before purchasing the drug.
Currently, nonprescription drugs, also known as over-the-counter (OTC) drugs, can be purchased without first seeing a doctor. OTCs cover everything from cold medicine to aspirin to supplements and can treat a wide variety of issues, from athlete’s foot to headaches. Nonprescription drugs are required to be labeled such that the consumer has enough information to self-select and administer the medications without the aid of a health care provider. For the most part, nonprescription drugs do not treat chronic health issues, and this is where the FDA sees an opportunity to expand the category of nonprescription drugs.
Drugs that treat chronic conditions are generally prevented from attaining nonprescription status because their labels do not contain enough information for the consumer to self-select and administer – either because the drug effects are more complex than can be conveyed in a label, or because they require monitoring by a physician. The proposed rule would require potential nonprescription drugs that wouldn’t need monitoring by a physician to have an ACNU as part of their new drug application. These ACNUs would provide an additional layer of information to help consumers determine if the drug is right for them. This information could be provided through an app or a phone call, or other similar methods of communication. The FDA estimates the proposed rule would, on average, save the consumer $26.70 per drug purchase by reducing the interaction between the consumer and the health system, and would save applicants for new drugs $55,469 per application by reducing the number of meetings between the FDA and industry. The FDA also predicts savings for the government and private payers, though it was not able to provide an estimate for those savings.
All in all, this rule is a common-sense win-win-win. Consumers, manufacturers, and government and private payers would all save time and money. Consumers would no longer need to see a doctor before purchasing a medication that is easy to self-administer and doesn’t require monitoring. There’s an added bonus: It’s quite possible this rule could make consumers a bit healthier overall. After all, studies show that even small barriers to obtaining medications make people less likely to take the medications they need. Manufacturers would spend less time haggling with other players in the prescription drug market, reducing red tape and therefore the cost of drugs. Government and private payers would save money by not having to haggle with manufacturers and by reducing unnecessary interactions between doctors and patients in need of prescriptions. There are also the potential downstream effects of patients being healthier and using services less if they’re more compliant with their medications. In short, the FDA’s proposed rule has real potential to both improve patient outcomes and lower costs.
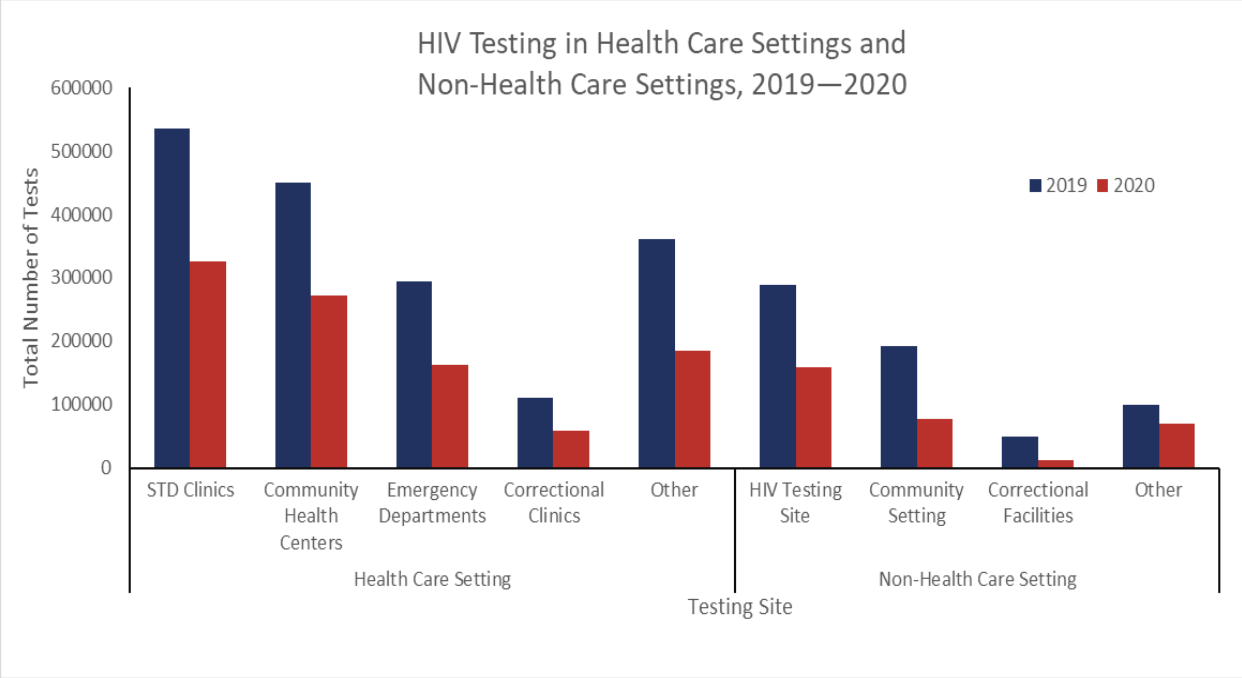
Chart Review: COVID-19 Impact on HIV Testing
Evan Turkowsky, Health Care Policy Intern
On June 24, the Centers for Disease Control and Prevention (CDC) released its report on how the COVID-19 pandemic disrupted HIV testing, which is key to addressing the HIV epidemic in the United States. From 2019 through the end of 2020, the number of CDC-funded HIV tests conducted decreased by over 43 percent. As shown in the chart below, the most common sites for HIV testing are health care settings—specifically, sexually transmitted disease (STD) clinics and community health centers, which respectively conducted 24.5 percent and 20.6 percent of all tests in 2020. These health care settings both experienced a nearly 40 percent decrease in the number of tests conducted from 2019–2020, while other testing sites, including correctional facilities and non-health care community settings, experienced the largest percent decreases in the number of tests distributed, at 73.7 percent and 59.8 percent, respectively. Notably, while overall testing declined, there was still an increase in the proportion of newly diagnosed individuals with HIV from a 0.4 percent positivity rate in 2019 to 0.5 percent in 2020.