Weekly Checkup
March 13, 2020
BARDA and the Coronavirus Vaccine Race
As of this week, the number of diagnosed novel coronavirus (COVID-19) cases surpassed 1,000 in the United States. Amid understandable anxiety, some on the left have taken to heart former Congressman (and White House Chief of Staff) Rahm Emanuel’s famous edict, “You never want a serious crisis to go to waste.” Democratic lawmakers have called on the Trump Administration to impose price controls on tests, treatments, and vaccines related to COVID-19, and they are framing their calls for broader drug-price regulation (as well as paid family medical leave and Medicare for All) in terms of the current outbreak.
Before Congress or the administration imposes drastic measures, it is worth examining what the federal government is already doing to encourage a vaccine for the outbreak. If history is any guide, the government’s current policies for supporting the emergency development of vaccines will likely be enough to ensure treatments are widely accessible.
The Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response in the Department of Health and Human Services, is currently soliciting proposals for the development of medical countermeasures for COVID-19. BARDA focuses broadly on investing in innovation, advanced research and development, and manufacturing medical countermeasures through funding, technical assistance, and services such as research networks. BARDA is also tasked with managing the transition of products from development through the Food and Drug Administration (FDA) approval process and then inclusion into the National Stockpile. As a connecting force between multiple federal agencies, BARDA provides a path to accelerating public-private partnerships for COVID-19 vaccine research and development.
Earlier this month, BARDA issued an announcement requesting proposals for COVID-19 diagnostics, vaccines, and therapeutics through a single point of entry on their website. They offer funding for selected projects within as little as 30 days. As of March 9, 2020, BARDA selected its first COVID-19-related product for development: a molecular diagnostic test from Hologic, Inc. BARDA plans to provide $699,000 to accelerate the development of this test. Similarly, BARDA has partnered with Janssen Research and Development of Johnson & Johnson, agreeing to share research and development costs in order to move along a COVID-19 vaccine to clinical evaluation. BARDA also plans to provide expertise and funds to Sanofi Pasteur, the vaccines department of pharmaceutical giant Sanofi, to support its vaccine candidate that uses an egg-free, recombinant DNA platform.
The expense and lag time involved in vaccine development are often impediments to rapid development, so the federal government’s most critical role is in helping push vaccine development forward. BARDA agreements do not typically involve explicit price restrictions, but in past public health emergencies, BARDA-funded treatments have been made available to the public at no charge. In the case of 2009 H1N1 vaccine, the federal government purchased doses and made them available to the public.
While the federal government is spending money at the early stages of development, this approach is not its only option. An alternative solution could be “pull incentives.” Sometimes called “challenge prizes,” these would encourage market entry and reward research after the fact, rather than paying costs up-front. Payments could be tied to lower prices, and such incentives would ensure drug prices remain low while still giving the private sector powerful incentives to invest in vaccine research. Pull incentives often include downstream financial rewards after approval for a certain product, so in combination with Emergency Use Authorizations from the FDA that allow companies to bypass FDA approval in the face of a public-health emergency, these pull incentives could apply to COVID-19 products.
While ensuring widespread access to any COVID-19 vaccine is clearly a priority, first policymakers and civil servants must work to ensure such vaccines are available at all. Turning the current crisis into a justification for widespread price controls would ultimately be self-defeating.
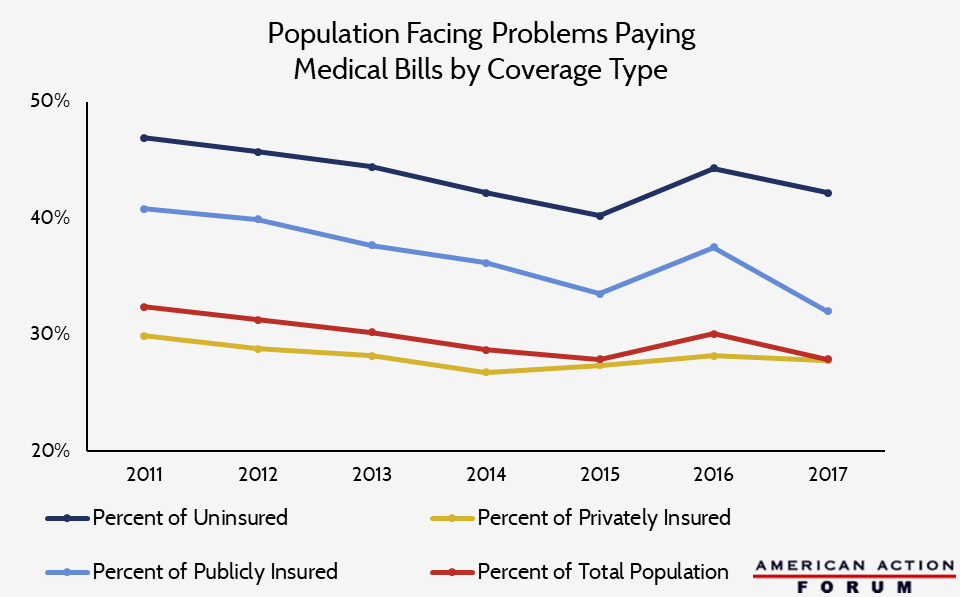
Chart Review: Insurance and Ability to Pay Medical Bills
Data obtained from National Health Interview Survey through Shadac State Health Compare
From Team Health
Daily Dish: Do We Need a Coronavirus Stimulus? – AAF President Douglas Holtz-Eakin
It is premature at best to pull the trigger on an ill-designed $1 trillion check-writing effort, given the constraints on government targeting and the limited information available.
Estimating the Cost of a Federal Paid-Leave Benefit During COVID-19 Outbreak – Labor Market Policy Data Analyst Isabel Soto and Director of Human Welfare Policy Tara O’Neill Hayes
The potential costs of an emergency paid-leave program are sizeable: A 14-day wage replacement benefit for all but the top 25 percent of earners could cost as much as $180 billion, depending on its generosity and targeting.
Podcast: The Long-Term Care Crisis – Director of Human Welfare Policy Tara O’Neill Hayes
The looming crisis of long-term care has several factors driving it, and current proposals to address it are not fiscally sustainable.
Worth a Look
Health Affairs: Antibiotic Resistance And Animal Consumption: The Case For Duration Limits In Food-Producing Animals
New York Times: Haunted by a Gene