Weekly Checkup
November 6, 2020
HHS Gives Regulatory Review Enforcement Some Teeth
One of the more significant undertakings of the Trump Administration has been its deregulatory push. In particular, it has required that each agency offset any new significant regulation by repealing two old ones, and it has aggressively sought to reduce the economic burden of the regulatory state. And by most metrics it has succeeded, making its deregulatory agenda, along with reshaping the federal courts and the Tax Cuts and Jobs Act, one of the major accomplishments of this term. It’s fitting, then, that this week the Department of Health and Human Services (HHS) published a new proposed rule that would sunset most regulations promulgated by any of the department’s agencies after 10 years unless the agencies review those rules.
AAF’s Director of Regulatory Policy Dan Bosch has an excellent write up of this proposed rule that you can read here. As Bosch explains, every president since Jimmy Carter has issued executive orders aimed at getting federal agencies to undertake retrospective reviews of existing regulations to determine if they are achieving their goals and if economic forecasts were accurate. Further, Congress enacted the Regulatory Flexibility Act in 1980, which also requires this review. But Bosch points out that there has been insufficient compliance with the statute or the executive orders over time. With its proposed rule, however, HHS is addressing this noncompliance head on by giving enforcement some teeth.
Under the new proposed rule, regulations would automatically expire after 10 years if no review has been conducted. Agencies would then have to go through the onerous process of repromulgating the rule if it was deemed crucial. According to Bosch, HHS anticipates reviewing over 2,000 rules during the first two years before settling into a pattern of examining roughly 125 rules a year thereafter.
Of course, the fate of this proposed rule rests at least in part on the outcome of the ballot counts currently ongoing in Pennsylvania, Nevada, and other states. Due to the 60-day comment period, HHS cannot finalize the rule until after January 5, 2021, and as Bosch points out, it would be difficult for HHS to publish a final rule prior to the inauguration. Even then the new rule could be subject to the Congressional Review Act, and it seems highly unlikely a Biden Administration will pursue the proposed rule.
That doesn’t mean the rule is a bad idea, however. Regardless of the ultimate fate of this proposed rule, and particularly in light of the many burdensome and unnecessary regulations that have been temporarily waived due to the pandemic, enforcing a regular review of federal regulations to make sure they are serving a useful purpose is an idea whose time has come.
Chart Review: Changes in Affordable Care Act Premiums
Julia Demeester, Health Care Policy Intern
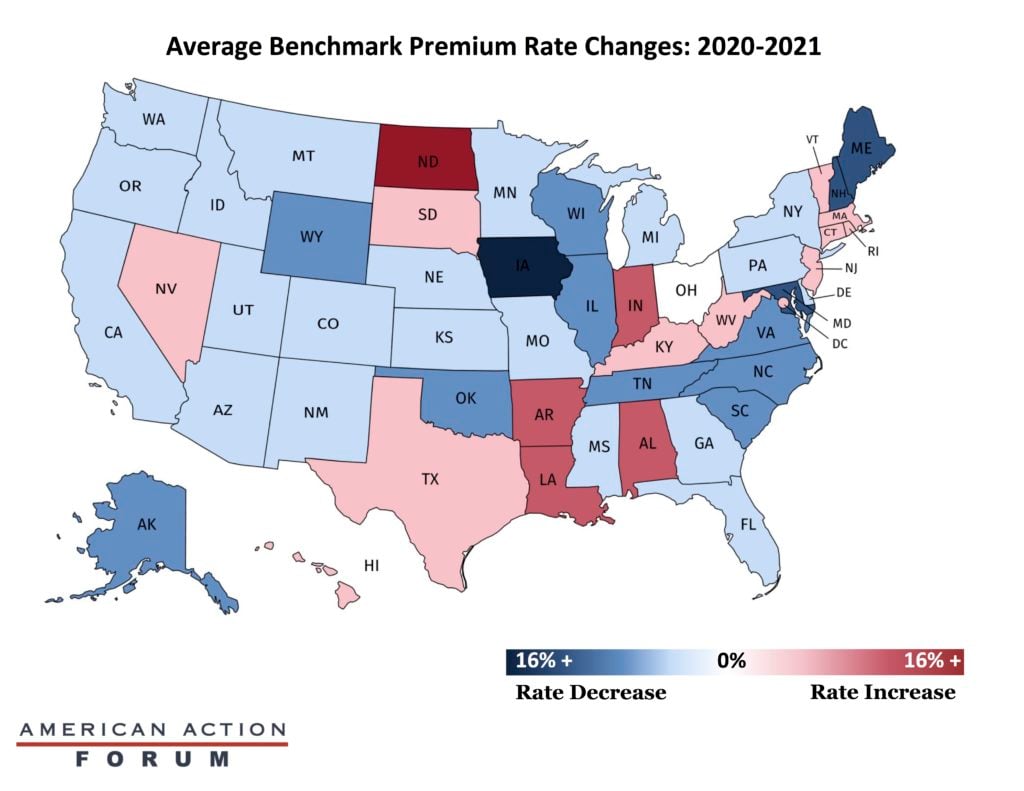
Insurers have released premiums and other information for the plans offered on the Affordable Care Act’s exchanges, and these data indicate that rates overall have dropped: Nationally, premium rates have decreased by roughly 2.16 percent between 2020 and 2021. This national average masks substantial variation between the states, as the map below depicts. Most states saw a decrease in premium rates from the previous year, although some did see an increase. North Dakota and Iowa were the two states with the most drastic changes. North Dakota saw a 28.7 percent increase in premium rates, while Iowa saw a 29.5 percent decrease. Ohio, in contrast, saw no change from the previous plan year. It is unclear how the pandemic is affecting the calculations of insurers, but it is likely that insurers are planning for higher health care costs in 2021 as people return to doctor’s offices for care they have delayed, receive a COVID-19 vaccine, or receive testing for the virus.
Data from the Kaiser Family Foundation
Worth a Look
Kaiser Health News: How Escalating COVID Cases Forced One State to Change Its Masking Strategy
Reuters: Aspirin to be tested as potential COVID-19 drug in UK study