Weekly Checkup
June 13, 2025
Putting 340B Where It Probably Should Have Been All Along
The President’s Fiscal Year 2026 Budget has catalyzed fervent discussion about the future of the 340B Drug Pricing Program (340B). Specifically, the budget proposed to transfer oversight of 340B from the Health Resources and Services Administration (HRSA) to the Centers for Medicare and Medicaid Services (CMS) – a reorganization that would represent the most significant structural change to the program since its creation in 1992.
The 340B Program, designed to offer steep drug discounts to safety net providers – including disproportionate share and critical access hospitals, rural health centers, and federally qualified health centers – has grown substantially since its inception, reaching more than $66 billion in drug spending in 2023. 340B’s rapid expansion, combined with recurring litigation and industry tension over program transparency, has intensified scrutiny. For example, manufacturers such as Eli Lilly and J&J have legally challenged HRSA over rebate-based discount systems within 340B, citing the need for tighter compliance and oversight to ensure that manufacturers’ rebates to hospitals are in fact going to needy patients, as the program was intended.
While the president’s proposed reorganization would operationally be a departure from the current structure, it would not change the governance or administration of the program. The proposal stipulates that CMS would receive the same $12-million budget that HRSA currently is allocated for overseeing 340B. CMS has historically been involved in 340B indirectly, particularly regarding deconflicting reimbursement policies between Medicaid and the program that affect how providers are paid for drugs.
Consolidating CMS’ broader health care financing and data infrastructure with 340B oversight may lead to a more unified regulatory framework, a reduction of redundancy, and an enhancement of program alignment with broader federal payment systems. While – as previously noted – there is nothing in the budget to suggest an imminent change, this reorganization does provide for some potentially impactful program updates.
Reaction to the president’s proposal has been relatively muted, likely due to its being just that – a proposal from the president rather than a formal proposal from the Department of Health and Human Services or legislation from Congress. Yet organizations that rely on 340B to sustain operations have decried this reorganization as a potential choke point and are claiming it would undermine program operations.
This argument is unfounded. CMS’ oversight and engagement could, in fact, positively influence how 340B drug pricing interacts with Medicare and Medicaid reimbursements. A more centralized approach could alleviate provider burden – a frequent complaint – and allow a more unified reimbursement structure under the occasionally competing and exclusionary public programs. Given the as-of-yet untested interaction with the Inflation Reduction Act’s (IRA) drug negotiation program – a separate travesty – unifying 340B with the IRA’s regulatory monstrosity might make sense to ensure that as long as both programs are operational, an entity isn’t double-dipping. CMS already possesses the necessary compliance and auditing mechanisms. The agency has stronger data capabilities, program interoperability pathways, and regulatory enforcement options at its disposal. These strengths could improve the program and better align it with its stated intent.
The president’s proposed transfer of 340B oversight to CMS marks a turning point for a program that has long operated in a way many believe is counter to its established parameters. As Congress considers the budget, it should embrace policy changes to ensure that the program’s original mission – to support access to affordable care – is met.
Chart Review: The Impact of Vaccines on Vaccine-preventable Diseases
With the Trump Administration’s announcement this week of the “retirement” of all members of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP), it is a good time to remind everyone about the benefits of vaccination.
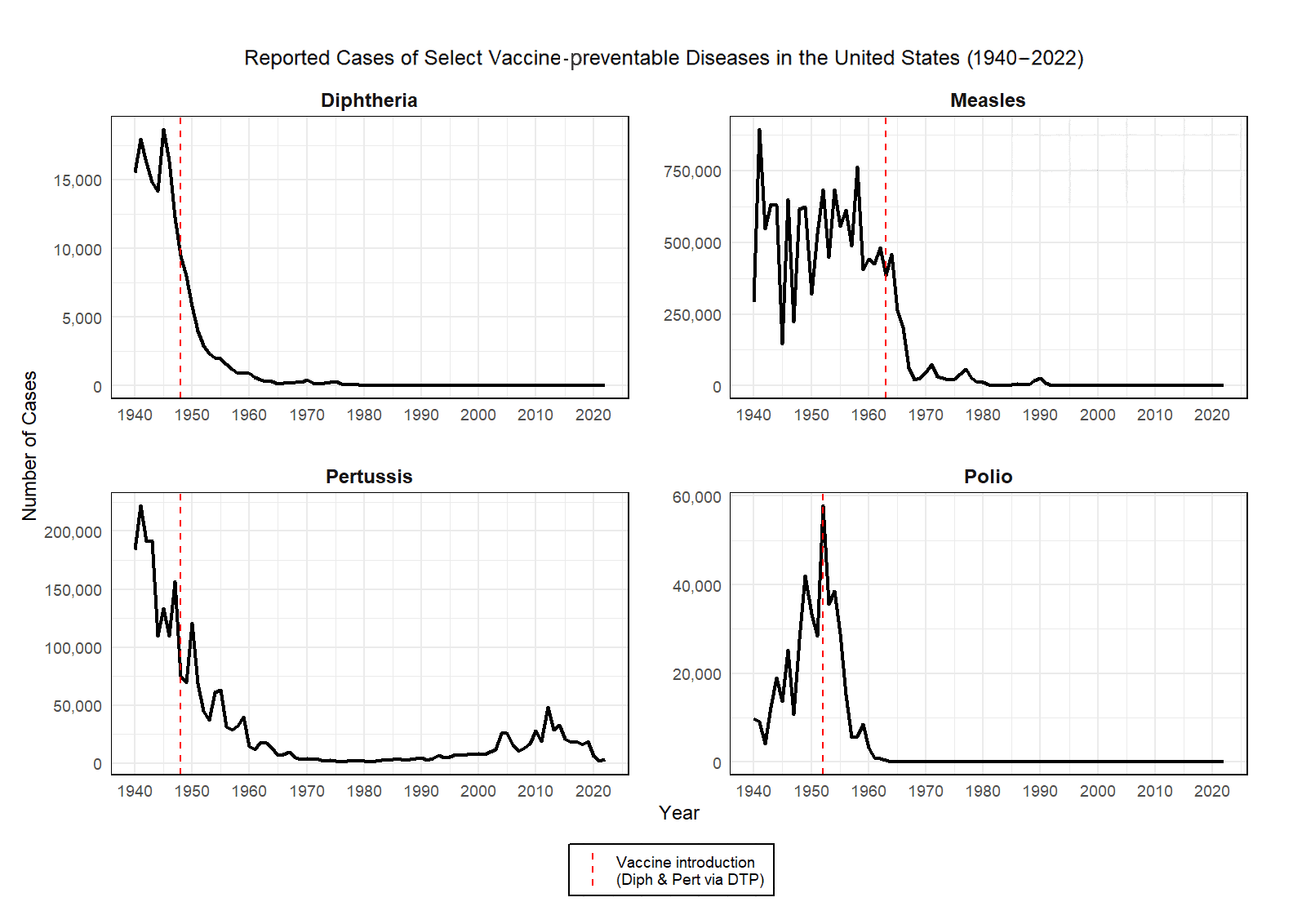
Vaccines have had a transformative impact on public health by dramatically reducing the prevalence of vaccine-preventable diseases across the globe. In the United States, widespread immunization programs have led to the near-elimination of diseases that once caused significant morbidity and mortality. For example, before the introduction of the measles vaccine in 1963, an estimated 3 to 4 million people contracted measles annually in the United States. Today, cases number in the hundreds, largely among unvaccinated populations. Similarly, polio, which paralyzed over 15,000 Americans each year during the early 1950s, has been eliminated domestically since 1979 thanks to aggressive vaccination efforts.
The chart below shows select vaccine-preventable diseases and what happened to the case numbers after the introduction of a respective vaccine. These data, which are compiled from the CDC, Public Health Reports, and the U.S. Census Bureau, clearly show the epidemiological benefit of getting vaccinated.
To learn more about the value of vaccines, including the economic benefits, see my recently published paper, “Vaccine Protection and Productivity: The Economic Value of Vaccines.”
Chart by Parth Dahima, Health Care Data Analyst