Weekly Checkup
January 11, 2019
The Shutdown & Federal Health Programs
The ongoing partial shutdown of the federal government doesn’t affect most federal health programs, because the bulk of the government’s health care activities are covered by appropriation bills that were signed into law last year. In fact, only about a third of the government is actually shut down.
The shutdown is, however, having an impact on a few important government functions in the health care space. First, the Indian Health Service (IHS) is funded not through appropriations for the Department of Health and Human Services (HHS) but through the Department of the Interior, which is caught up in the shutdown. That means American Indians and Alaska Natives, who depend on these facilities, will be going without health services in the very near future. So far the IHS has been able to continue operations using carry-over funding, but those resources will begin to run out shortly.
Second, the Food and Drug Administration (FDA)—though part of HHS—receives its appropriated funding through the Agriculture, Rural Development, Food and Drug Administration, and Related Agencies appropriations bill, and that funding package is part of the one-third of federal spending that hasn’t been provided. As a result, much of the FDA’s domestic food-safety inspections, though not all, have halted. On the other hand, inspections of foreign food imports have been deemed essential and are continuing. Like the IHS, the FDA can also continue nonessential operations using remaining 2018 funds while they last.
Unlike other federal agencies, however, most of the FDA’s funding doesn’t come from federal appropriations. Instead, the FDA’s brand-name and generic pharmaceutical, medical device, and biologic and biosimilar review and approval processes are funded through industry user fees (read AAF’s primer on the user fees for more detail). It gets even more complicated: The FDA can’t accept new user-fee payments during the shutdown, but it can continue operations for which user fees have already been paid. Additionally, the FDA is shifting around existing user-fee monies to prioritize post-market drug safety surveillance over new drug approvals. All told about 59 percent of FDA’s workforce is still going about its business as the agency juggles available funding and critical responsibilities to try to maintain food and drug safety.
The shutdown could also have long-term implications for both the FDA and IHS. The longer the shutdown continues, the more likely it is that the IHS fails to meet critical health needs or that contaminated food slips past the FDA’s reduced inspections. And the FDA already faces backlogs in its device and drug approvals. To the degree new applications pile up, or work is slowed or stopped on existing applications, the backlog will only worsen.
Chart Review
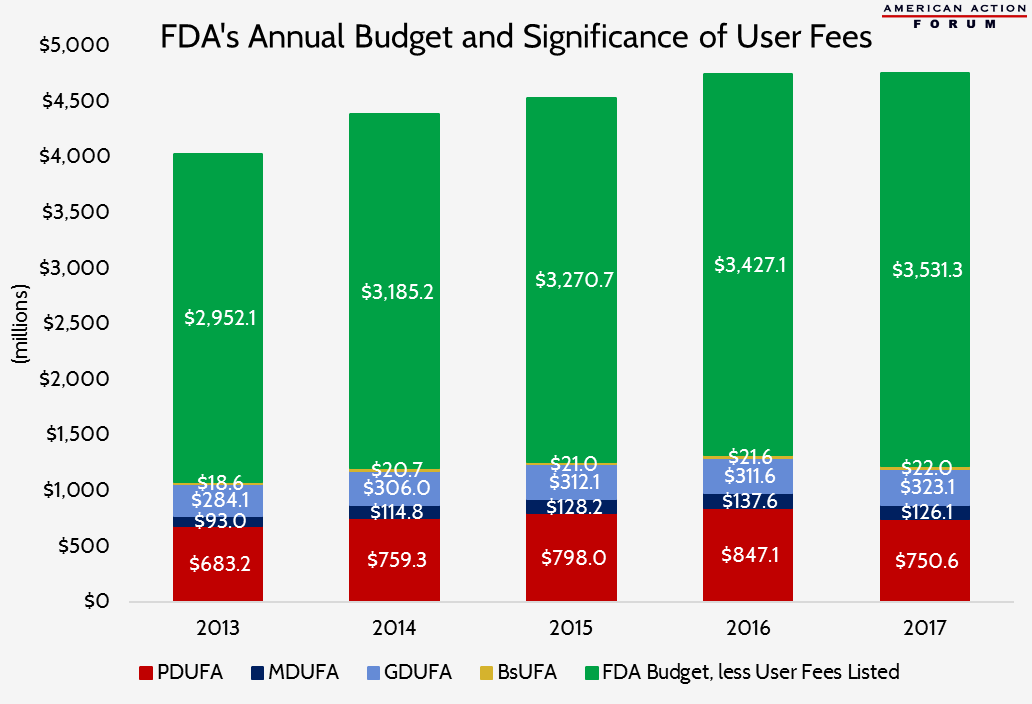
In 2017, AAF examined the Food and Drug Administration’s user fees. The following chart shows the significance the prescription drug and medical device user fees play in FDA’s annual budget. These four user fees alone accounted for 27 percent of the FDA’s budget from 2013 to 2017.
Worth a Look
Axios: The Senate’s plan to tackle drug prices
Health Affairs: The Earned Income Tax Credit, Poverty, and Health