Weekly Checkup
August 28, 2020
Mixed Signals on COVID-19 Testing
Last week’s edition of the Weekly Checkup considered the Food and Drug Administration’s (FDA) recent emergency authorization for a new, quick, low-cost COVID-19 test, and the reality that a vaccine by the end of the year won’t be a panacea that returns everything to “normal” in the blink of an eye. Effective, widespread testing that allows public health officials, school administrators, and employers to quickly identify cases of COVID-19 and act to mitigate spread is critical to any near-term return to normal. This week, the FDA announced emergency authorization of yet another cheap and fast-acting COVID-19 test while at the same time the Centers for Disease Control and Prevention (CDC) confusingly sought to discourage testing of pre- and asymptomatic individuals. Let’s discuss.
First consider the news that Abbott Pharmaceuticals will be rolling out its latest rapid test in September at a cost of only $5 per test. Unlike the test developed by Yale researchers, this test doesn’t require a lab, and while it’s intended to be administered by a medical professional, that professional could be a school nurse, or a pharmacist, or even say a nurse hired by an employer to administer screenings as part of preventing spread in an office or factory environment. And because the test doesn’t require a lab, it’s especially quick, with results in 15 minutes. This test is also different from the Abbott rapid test that has been used by the White House and others, which had a high rate of false positives. Instead the new BinaxNOW COVID-19 Ag Card test is 97 percent accurate. Abbott has been working for some time to expand its manufacturing capacity and is aiming to deliver 50 million of the new rapid tests per month to the U.S. market by October, which is staggering considering that to-date, in total, the U.S. has only conducted 74 million tests. Additionally, the Trump Administration last night announced an agreement with Abbott, to purchase 150 million of these tests at a cost of $750 million—or exactly $5 a test—for distribution to high-risk locations such as nursing homes or schools and universities.
While the administration is, and should be, touting this development, there was also news this week about changes to CDC’s recommendations on testing. The agency is now advising against testing people who are not symptomatic of the virus, even if they’ve experienced exposure. This might have made sense earlier in the pandemic when testing capacity was particularly limited, but it is strange when more and more testing options are coming online. The Trump Administration hasn’t exactly been successful in explaining the rationale behind the decision, though it should be noted the FDA’s emergency use authorization for Abbott’s new test only allows for use on patients who are symptomatic at this time, so some concerns over testing capacity might remain. At the same time, some are openly wondering if this policy change isn’t in keeping with the president’s stated desire to see less testing, which he blames for the increase in reported cases of COVID-19. Whether this is the case or not, failure to test pre- and asymptomatic individuals will make it harder to stem the pandemic and return to some semblance of normal life. Symptomatic individuals know they’re sick, even if they aren’t certain they have coronavirus, so they can isolate themselves to prevent the risk of spreading it, but transmission is most problematic in the pre-symptomatic stage. There is also increasing evidence of asymptomatic transmission, though data on this facet of the pandemic have been mixed.
Until we have a vaccine and/or a highly effective treatment, widespread and regular testing of non-symptomatic individuals, particularly those who are known to have had exposure, will be critical to returning to school and work. Even with a vaccine, a robust testing regime will be a necessary compliment for at least the first year and possibly longer. Perhaps the CDC will provide solid justification for the new guidelines, but discouraging testing, just at the moment we are ramping up testing capacity, seems counterproductive at best.
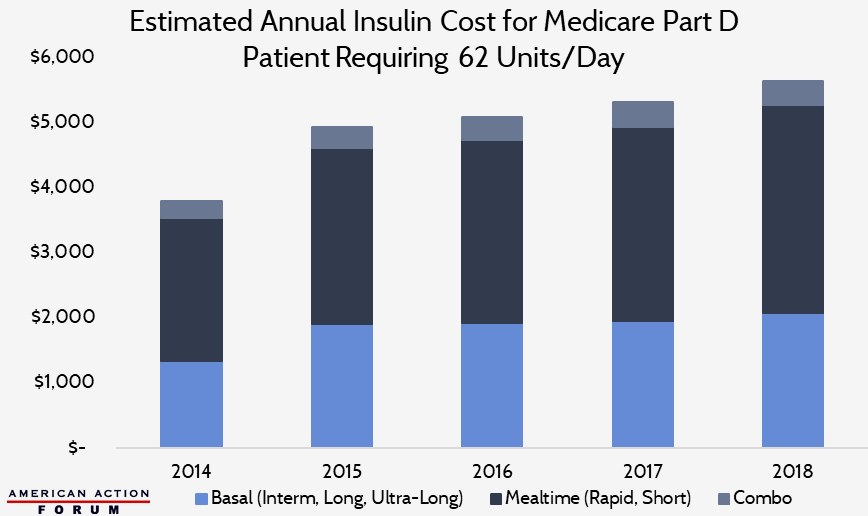
Chart Review: Estimated Annual Insulin Cost for Medicare Part D Patient
Josee Farmer, Former Health Care Policy Intern
The Health Care Cost Institute estimates that the average insulin user requires 62 units per day. This estimate includes 22 basal units, which are long or ultra-long acting; 36 mealtime units, which are rapid or short acting; as well as 4 units of combination insulin. The chart below depicts the total annual cost of insulin for a patient facing a typical dosage requirement. From 2014 to 2015, the average amount spent on basal units increased substantially – largely attributed to the introduction of ultra-long-acting insulin to the market in 2015. By 2018, the average annual cost for a typical insulin user surpassed $5,600 in the Medicare Part D market. The same trend can be seen in the Medicaid and employer-sponsored insurance markets, as well.
Data obtained from CMS Medicare Part D Drug Spending Dashboard
Worth a Look
Reuters: Scientists map out mosquito immune system to help fight malaria
Kaiser Health News: Prognosis for Rural Hospitals Worsens With Pandemic