Weekly Checkup
October 26, 2018
The Trump Administration’s Very Busy Health Policy Week
Let’s recap the Trump Administration’s health-policy proposals from the week: first, on Monday, regulatory revisions to the Affordable Care Act’s (ACA) section 1332 waivers; second, on Tuesday, a proposed rulemaking seeking to expand heath reimbursement arrangements (HRAs); finally, on Thursday, an effort aimed at curbing the cost of physician-administered medications. Let’s take these one at a time.
Monday: Section 1332 of the ACA allows states, under certain conditions, to obtain waivers from ACA requirements in order to experiment with alternatives that might better fit their populations. This AAF primer explains the particulars, but the important thing to know is that the waivers haven’t gotten a lot of use. That’s mostly due to the Obama Administration’s restrictive interpretation of the statute that amounted to: You can do something different than the ACA, as long as it’s exactly like the ACA. The Trump Administration’s new regulatory guidance, released this week, seeks to give states more flexibility. AAF’s Tara O’Neill Hayes has written on the details of the new guidance here. The biggest controversy coming out of the new guidance has been its supposed rollback of protections for those with preexisting conditions. As AAF President Doug Holtz-Eakin explained in Tuesday’s Daily Dish, that claim is disingenuous because, to get a waiver, states would have to show that plans that comply with ACA coverage and affordability requirements would still be available.
Tuesday: As AAF’s Jonathan Keisling explains here, pre-ACA HRAs allowed employees to use tax-preferred dollars to purchase individual market insurance or a la carte health care services. The ACA’s rules resulted in HRAs mostly disappearing from the insurance market. The Trump Administration is proposing changes that would allow the use of HRAs for the purchase of ACA-compliant health insurance (aka Qualified Health Plans, or QHPs) in the individual market. As proposed, employers would be restricted in how and to whom they offer HRAs to prevent them from dumping unhealthy employees into the individual market—which could upset the risk pool. The Department of Treasury’s analysis of the proposed rule finds that it could increase enrollment in QHPs by 10 million people by 2024, adding stability to the individual market and potentially reducing premiums. Check out AAF’s complete analysis for more details.
Thursday: Finally, President Trump announced his administration’s intention to develop a new initiative aimed at curtailing spending on pharmaceuticals. Specifically, he wants to bring Medicare spending on physician-administered medicines through the Part B program in line with lower spending on the same medications overseas. The administration is selling this proposal as a market-based reform. To be fair, there is need for reform, as the current system in certain instances encourages doctors to use higher-priced drugs. But current Part B payments are based on the average price paid in the private market inclusive of all discounts and rebates provided for those drugs. In other words, the existing Part B reimbursement model is market-based, as this AAF primer explains in detail. In essence, the administration is proposing a move toward price controls. Fixing prices to prices fixed abroad is, well, price fixing. Further, basing that fixed price on the fixed prices of foreign, socialized health care systems can hardly be framed as an embrace of free markets. It’s also worth noting that the degree to which this proposal would improve incentives and drive savings is dependent on the success of new third-party drug purchasing vendors. Those vendors sound an awful lot like pharmacy benefit managers (PBMs)—odd, given the administration has blamed PBMs for being a primary driver of excessive drug spending.
Chart Review
Jonathan Keisling, Health Care Policy Analyst
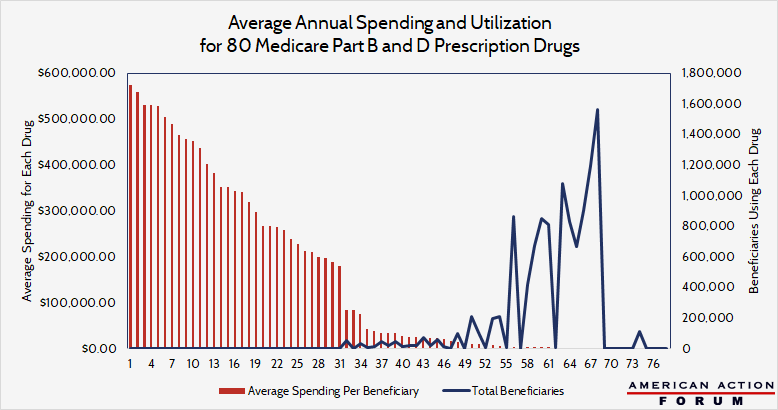
In 2016, 14 percent of Medicare spending was on prescription drugs, and, as the chart below shows, utilization rather than extremely high drug costs is driving this spending. The chart overlays the data for utilization and cost per user for the top 80 drugs by cost and use. For this analysis, 80 drugs were chosen, 40 for each program, based on the following criteria: top 15 for toal spending, top 15 for spending per user, and/or the top 10 highest annual unit cost increases. These 80 drugs were used by 11 million beneficiaries and cost Medicare roughly $47 billion in 2016—yet the bulk of that spending was on drugs that cost relatively little. Only 255 people used the most expensive drug, with total spending on that drug in 2016 totaling $570,000 per beneficiary—making up 0.3 percent of the total annual cost of these 80 drugs. In contrast, the most used drug comprised over 5 percent—1.6 million people took the most used drug, with per beneficiary spending on that drug totaling $1,500 (which works out to $8 per dose).
From Team Health
Trump Administration Offers States More Flexibility on Health Insurance
Deputy Director of Health Care Policy Tara O’Neill Hayes explains the administration’s proposal to make it easier for states to apply for waivers from Affordable Care Act rules.
Sizing Up The Proposed HRA Rule
Health Care Policy Analyst Jonathan Keisling digs into the Trump Administration’s proposal to make it easier for employers to offer Health Reimbursement Arrangements (HRAs).
Eakinomics: Trump’s Drug Price Initiative
AAF President Douglas Holtz-Eakin assesses President Trump’s proposed plan to bring down the cost of drugs in Medicare Part B.