Weekly Checkup
September 18, 2020
Cutting Through the Vaccine Headlines
There’s been a lot of news and competing claims in recent weeks about COVID-19 vaccines. Questions about when they’ll be available, whether they’ll be safe, and just how soon this pandemic will be over are mixing with election-year politics, and the result is confusing and unsettling. But the Weekly Checkup is here to try to make sense of it all. The bottom line is that any vaccine that gets approved will likely be safe, but it won’t immediately solve our health or economic crisis.
The first thing to keep in mind is that a vaccine will be considered a success even if it is only 50 percent effective at preventing recipients from contracting COVID-19, according to National Institute of Allergy and Infectious Diseases Director Dr. Anthony Fauci. Perhaps that’s why Centers for Disease Control and Prevention Director Robert Redfield said this week in a congressional hearing that wearing a mask could still offer more protection against contracting COVID-19 than a vaccine once one or more are widely available. A vaccine will be an important tool in fighting COVID-19, but it won’t end the pandemic on its own.
Second, despite the president’s promises, a vaccine rollout won’t happen overnight. There is a growing possibility a vaccine will receive emergency use authorization from the Food and Drug Administration (FDA) before the end of the year. Pfizer CEO Albert Bourla said this week that his company hopes to have data from its Phase 3 clinical trial of a vaccine candidate to the FDA by the end of October. At the same time, however, as Director Redfield said this week and Dr. Fauci has said previously, there is no feasible way to roll out a vaccine widely before the end of the year. Best case scenario, we’re looking at mid to late 2021 before a vaccine is available to the majority of the U.S. population.
The third area of concern for many is safety. No one can promise 100 percent safety, and the president’s constant cheerleading and haranguing of public health officials to get a vaccine out as soon as possible doesn’t help confidence. Neither does his public disputing with Drs. Redfield and Fauci this week over how long it will take to get a vaccine distributed to all Americans and the efficacy of mask wearing. The president’s electoral concerns appear to be mixing with his policy objectives in ways that could reasonably undermine public confidence in a vaccine’s safety.
But it won’t be the president who decides if a vaccine is safe. Last week the nine biotech companies that are actively involved in development of a COVID-19 vaccine released a joint statement promising to put safety first as they move forward with their research. All the companies pledged that they will not seek approval for a vaccine candidate unless it has “demonstrated safety and efficacy through a Phase 3 clinical study.” The reality is that while failure to deliver a vaccine would be a blow for any of these companies, putting a vaccine on the market that is harmful could be catastrophic. Additionally, FDA Administrator Stephen Hahn has repeatedly insisted that the FDA will not be pressured into approving an unsafe vaccine for political reasons, and comments by past FDA directors and public health officials indicate it is highly unlikely the FDA would be forced to approve a vaccine about which officials had concerns. The Secretary of Health and Human Services could overrule an FDA decision against a vaccine, but such an action would destroy public confidence in the vaccine and ultimately be self-defeating. If the FDA approves a vaccine, Americans should have a high degree of confidence that politics didn’t play a role. And if the FDA gets overruled, we’ll know about it.
There is a lot to be excited about on the vaccine front over the next year to 18 months, but it’s important not to get carried away. A vaccine could be coming soon, and it will help in the fight against COVID-19, but a vaccine won’t be a silver bullet that returns us to normalcy. Testing, treatment, and precautions like mask wearing and social distancing will continue to be crucial.
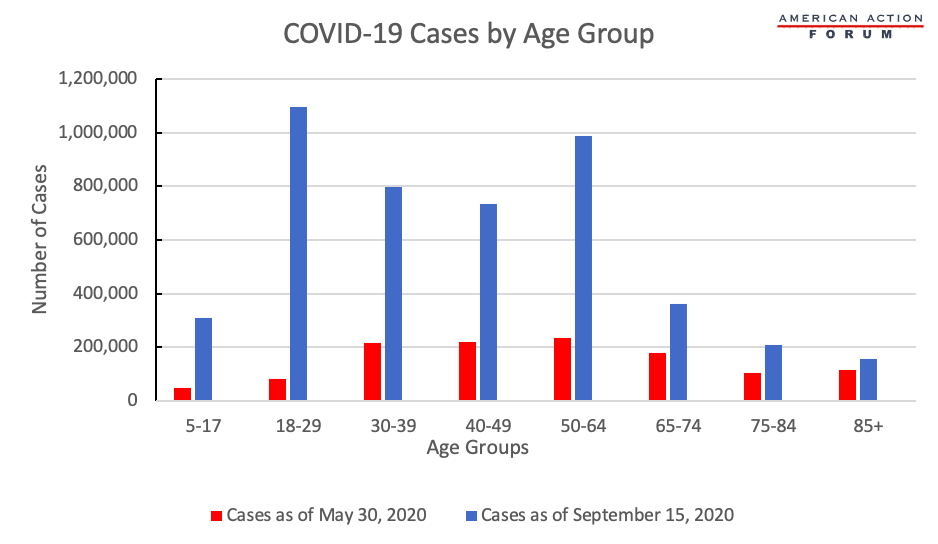
Chart Review: COVID-19 Cases Spike as Students Return to College
Julia Demeester, Health Care Policy Intern
In the early months of the pandemic, adults between the ages of 50 and 74 were disproportionately impacted by the coronavirus: As of May 30, 2020, those 50 to 64 years old had the highest rate of COVID-19 cases. Nearly four months later, that age group is still one of the most affected groups, but data from the Centers for Disease Control and Prevention (illustrated below) indicate a massive surge in cases among much younger demographics. In May, the number of cases among those 18 to 29 years old was 82,469. By September 15, 2020, the cases among this age group jumped tenfold to 1,096,268. The surge in cases among this age group is likely due to a number of reasons, such as summer vacations, but the return to college campuses has played an outsized role in the increase of coronavirus cases for this age group, despite the extensive efforts made by university officials.
Worth a Look
New York Times: An Experimental Drug Protects Covid-19 Patients, Eli Lilly Claims
Associated Press: Petalo, not Charmin: Virus brings Mexican toilet paper to US