Week in Regulation
April 17, 2023
EPA Proposal Leads Moderately Busy Week
Regulatory activity moved at a relatively steady yet modest beat last week. There was a baker’s dozen of rulemakings in the Federal Register that had some quantifiable economic impact. While a handful of these had effects measured in the hundreds of millions of dollars, none was necessarily earth-shattering on a stand-alone basis. A proposed rule from the Environmental Protection Agency (EPA) was the most significant of the bunch. Across all rulemakings, agencies published $1.1 billion in total costs and added 17,206 annual paperwork burden hours.
REGULATORY TOPLINES
- Proposed Rules: 70
- Final Rules: 71
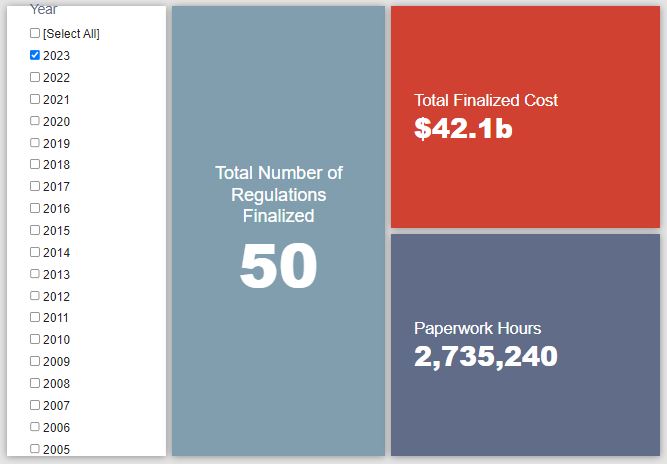
- 2023 Total Pages: 23,286
- 2023 Final Rule Costs: $42.1 billion
- 2023 Proposed Rule Costs: $57.9 billion
NOTABLE REGULATORY ACTIONS
The most consequential rulemaking of the week was the EPA proposed rule regarding “National Emission Standards for Hazardous Air Pollutants: Ethylene Oxide Emissions Standards for Sterilization Facilities Residual Risk and Technology Review.” Specifically, the proposal seeks to establish an updated set of standards regulating ethylene oxide (EtO) emissions from certain facilities that sterilize medical devices. EPA bases this action on its finding “that EtO is a far more potent carcinogen than EPA had understood at the time of the previous RTR [risk and technology review] for this source category.” The agency estimates that the proposed rule’s new requirements will impose $74 million in annual costs, or $640 million in total over a 20-year period.
TRACKING THE ADMINISTRATIONS
As we have already seen from executive orders and memos, the Biden Administration will surely provide plenty of contrasts with the Trump Administration on the regulatory front. And while there is a general expectation that the current administration will seek to broadly restore Obama-esque regulatory actions, there will also be areas where it charts its own course. Since the AAF RegRodeo data extend back to 2005, it is possible to provide weekly updates on how the top-level trends of President Biden’s regulatory record track with those of his two most recent predecessors. The following table provides the cumulative totals of final rules containing some quantified economic impact from each administration through this point in their respective terms.
![]()
For the first time in roughly a month, the Biden Administration saw some noticeable changes in its final rule totals, with increases in costs of $217.8 million and paperwork of 28,500 hours. A set of energy efficiency standards for air cleaners provided most of the cost bump. There was far less action from either of the other administrations. The Trump-era totals remained virtually unchanged. Meanwhile, Obama-era costs bumped upwards by roughly $20 million due to a set of airworthiness directives.
THIS WEEK’S REGULATORY PICTURE
This week, the Food and Drug Administration (FDA) proposes to open up its regulatory standards for “salt substitutes.”

Source: Photo by Peter Werkman on Unsplash
FDA has long established Standards of Identity (SOI) for Food “to ensure that the characteristics, ingredients and production processes of specific foods are consistent with what consumers expect.” This past week, the agency published a proposed rule that adjusts the standards involving one of the most ubiquitous ingredients possible: salt. More to the point, FDA is seeking to update the relevant regulatory language to “permit the use of safe and suitable salt substitutes to replace some or all of the salt used in the manufacture of standardized foods.”
As FDA notes, “salt is a required or an optional ingredient in 140 SOI.” Under the existing framework, however, “salt” is defined specifically as sodium chloride. Food manufacturers that would like to use a “salt substitute” in the production of any of these 140 categories of food are unable to do so under the current regulatory definitions. This proposed rule would go into all of these relevant provisions and update the language to allow affected manufacturers to “use substitute ingredients that provide similar taste and other technical functions of salt in foods.”
FDA is undertaking this rulemaking in the hopes of eventually reducing the overall consumption rate of sodium. The agency finds that the 3,400 milligrams of sodium per day consumed by the average American is dangerously excessive and leads to increased incidence of cardiovascular issues such as hypertension and stroke. Allowing manufacturers to utilize substitutes instead of merely forgoing salt altogether would presumably help lower that rate.
The agency largely discusses the rulemaking’s costs and benefits in qualitative terms, noting that:
We currently lack data on these potential industry responses and request public comment from manufactures, suppliers, and consumers on the extent to which the additional flexibility provided by this rule would be used by manufacturers, hence also desired or tolerated by consumers, and viable in the supply chain.
Interested parties have until August 8, 2023, to submit comments.
TOTAL BURDENS
Since January 1, the federal government has published $100 billion in total net costs (with $42.1 billion in new costs from finalized rules) and 13.9 million hours of net annual paperwork burden increases (with 2.7 million hours in increases from final rules).