Testimony
May 5, 2021
Lower Drug Costs Now: Expanding Access to Affordable Health Care
U.S. House of Representatives Committee on Education and Labor Subcommittee on Health, Employment, Labor, and Pensions
*The views expressed here are my own and do not represent the position of the American Action Forum. I am indebted to my colleagues Tara O’Neill Hayes and Christopher Holt for their continued efforts to educate on these issues.
Chairman DeSaulnier, Ranking Member Allen, and members of the Subcommittee, thank you for the opportunity to testify today on the matter of drug prices. I hope to make three basic points:
- The term “rising drug costs” is riddled with ambiguity; list prices, net prices, out-of-pocket prices, development costs, and total spending on drugs have displayed very different patterns over time.
- The provisions of Title I of H.R. 3 are far from a government “negotiation” and are a threat to the dynamic ecosystem that drives U.S. pharmacological innovation.
- A superior approach would be the reforms to Medicare Part D in H.R. 19, a superior version of the reform in Title III of H.R. 3.
Let me discuss these further.
Introduction
Over the past several years, the public’s attention has increasingly been focused on the cost of health care, and specifically the contribution of prescription medications to those costs. Policymakers, however, should first clearly identify the actual problem they’re trying to address.
Identifying the Problem: Patterns in Drug Costs
There is little consensus in the term “rising drug costs,” making it difficult to determine if there is an actual policy problem, its size, or its scope. The first step in identifying whether there is a problem is to differentiate between prices, costs, and spending, which are related but not identical.
For example, “rising drug costs” might refer to a narrow definition focused on the sales prices (or “list price”) set by drug developers and manufacturers. Alternatively, the problem might not be with all drugs, but instead the high prices of some drugs. Finally, the problem may be the increasing cost of prescription drugs borne by individuals at the pharmacy counter, which has resulted from an increase in high-deductible health plans and greater use of co-insurance, rather than flat co-pays.[1]
Rising drug costs could also mean an increase in overall prescription drug expenditures, whether in dollar figures or as a percentage of National Health Expenditures (NHE). Because spending is a function of both price and quantity, this could result from increased utilization due to rising national reliance on prescription drugs or broader access to them.
Pharmaceuticals as a Share of National Health Expenditures
The first important fact to consider is that prescription drug spending as a percent of NHE has remained steady at about 10 percent since 2000, the same percentage it was in 1960. There was a dip in prescription drug spending as a share of NHE in the years between 1960 and 1980, as advances in technology and expanded insurance coverage of hospital visits contributed to a shift in NHE towards hospital stays.[2] In the 1980s, that trend began to reverse as new pharmaceuticals became widely available for the treatment of many of the most prevalent diseases in American society. The availability of advanced pharmacological treatments is highly correlated with reduced expenditures for hospitals and other health professionals.[3] In fact, the Congressional Budget Office estimated in 2019 that increased use of prescription drugs would lead to twice as much in cost savings on hospital and physician services as it would increase costs on pharmaceuticals.[4] As pharmaceutical growth began to level out to roughly the same levels as the 1960s, so did other NHE categories.[5] Viewed from this national perspective, there appears to be little empirical support for a perceived radical rise in drug spending in the data, although national averages can mask the variance among subpopulations and the most current NHE data is more than a year old.
Drivers of Drug Spending
To the extent that drug expenditures are increasing or will begin to increase in the near future, a key factor is utilization. Annual growth in pharmaceutical spending in February 2020 was 7.9 percent,[6] but annual pharmaceutical price growth was only 2.4 percent.[7] On a per capita basis, real net spending has grown by only 1 percent between 2007 and 2017 and actually declined by 2.2 percent in 2017.[8]
Still, Americans are getting older, living longer, and are increasingly burdened with chronic disease. As of this year, 60 percent of the United States’ adult population had been diagnosed with at least one chronic health condition, and 40 percent had two or more chronic conditions.[9] Managing these chronic conditions is an expensive proposition that relies primarily on medication. Eighty-six percent of all health care spending is for patients with one or more chronic disease; 98 percent of Medicare and 83 percent of Medicaid spending goes toward providing care for the chronically ill.[10], [11] Specifically, over 75 percent of U.S. health care spending goes toward treatment of chronic disease.[12] As these trends continue, the financial burden of maintaining a high quality of life with chronic conditions will inevitably disproportionately increase the growth of pharmaceutical health care spending.
Drivers of Drug Prices
Developing new treatments is an expensive prospect in terms of both capital and time. A Tufts University study in 2016 found that the average cost for each drug successfully brought to the market is nearly $2.9 billion.[13] Data from the Organisation for Economic Co-operation and Development also show that the amount of spending per new drug approved has been growing for decades.[14] It takes an average of 15 years from the time a drug developer first begins testing a new formula until the Food and Drug Administration (FDA) approves it.[15] Only 1 in 1,000 drug formulas will ever enter pre-clinical testing, and of those, roughly 8 percent will ultimately receive FDA approval.[16]
Additionally, the last decade has seen a significant shift toward the use of “specialty drugs.” While there is no precise definition of a specialty drug, this term typically refers to drugs with at least one of the following characteristics: requires special handling, must be administered by a doctor, requires patient monitoring or follow-up care, or is used to treat complex, chronic conditions.[17] As a result, these drugs tend to be quite expensive.
In fact, by 2016, about half of the top 80 most expensive drugs nationally were specialty drugs, and that number is increasing annually.[18] In 2010, the United States spent just over $11.5 billion on the top 25 specialty drugs. By 2018, net spending on specialty medicines reached $170 billion, accounting for 49.5 percent of all expenditures on medicines, despite accounting for just 2.2 percent of the volume.[19] specialty drugs are often more expensive to develop and typically treat small patient populations with very specific and otherwise untreatable diseases, they tend to have higher prices. Over time, the cost of new specialty drugs per patient will likely continue to be higher as the target population for each new drug will grow smaller with the development of treatments for less common diseases.
List Versus Net Prices
An important aspect of the discussion is the difference between list price and net price. List prices for brand-name drugs, on average, increased between 5.2 and 9.3 percent between 2015 and 2019, yet the average net price of these drugs has grown between 0.3 and 2.9 percent, with the trend being flat or downward sloping.[20] In fact, price growth for prescription drugs over the course of 2018 was the lowest growth rate since 2013, and even dipped into negative territory between December 2017 and early 2018.[21] So while the average list price of brand name drugs rose 69 percent between 2010 and 2019, average out-of-pocket (OOP) costs for those drugs declined from $27.72 in 2015 to $26.25 in 2019.[22]Generic list prices have declined, on average, during this time period, and insured patient OOP costs have remained relatively unchanged.[23] The increasing difference between list and net price points to the growing use of discounts and rebates. Understanding the role of these incentives in price determination is an area worthy of careful consideration to ensure resources are being allocated as desired.
Out-of-Pocket Prices
From a patient perspective, many anecdotally report that OOP costs are climbing and the increased frequency of high-deductible health insurance plans is cited as the reason. But the data show that average patient OOP costs at the pharmacy counter have actually declined since 2013. Roughly 30 percent of all medicines were available in 2019 for zero OOP costs, and 90 percent were available for $20 or less, with the average OOP cost for insured patients equaling $10.67. Only 1.1 percent of prescriptions filled had a co-pay of more than $125. Uninsured patients have seen their OOP costs increase 38 percent, though only 29 percent of patients paying cash face costs greater $125.[24]
For the small share of very costly drugs, the expense adds up fast: 3.4 million prescriptions (0.1 percent of all prescriptions filled in 2017) had an OOP cost of more than $500, with an average cost of $1,502; total OOP expenditures for these drugs was $5.2 billion.[25] In 2019, 9.9 percent of patients paid more than $500 in annual OOP costs, including 2.3 percent who paid more than $1,500.[26] Seniors enrolled in Medicare Part D are most likely to have such high OOP costs, reflecting both their greater likelihood to take prescription medicines, as well as the program’s benefit design, namely the lack of an OOP cap. It is likely also true that a number of prescriptions that would have cost at least that much were never filled because the patient simply could not afford it (or chose not to spend the money). The abandonment rate for brand-name drugs reached 21 percent in 2017.[27]
Recent Developments in Drug Prices
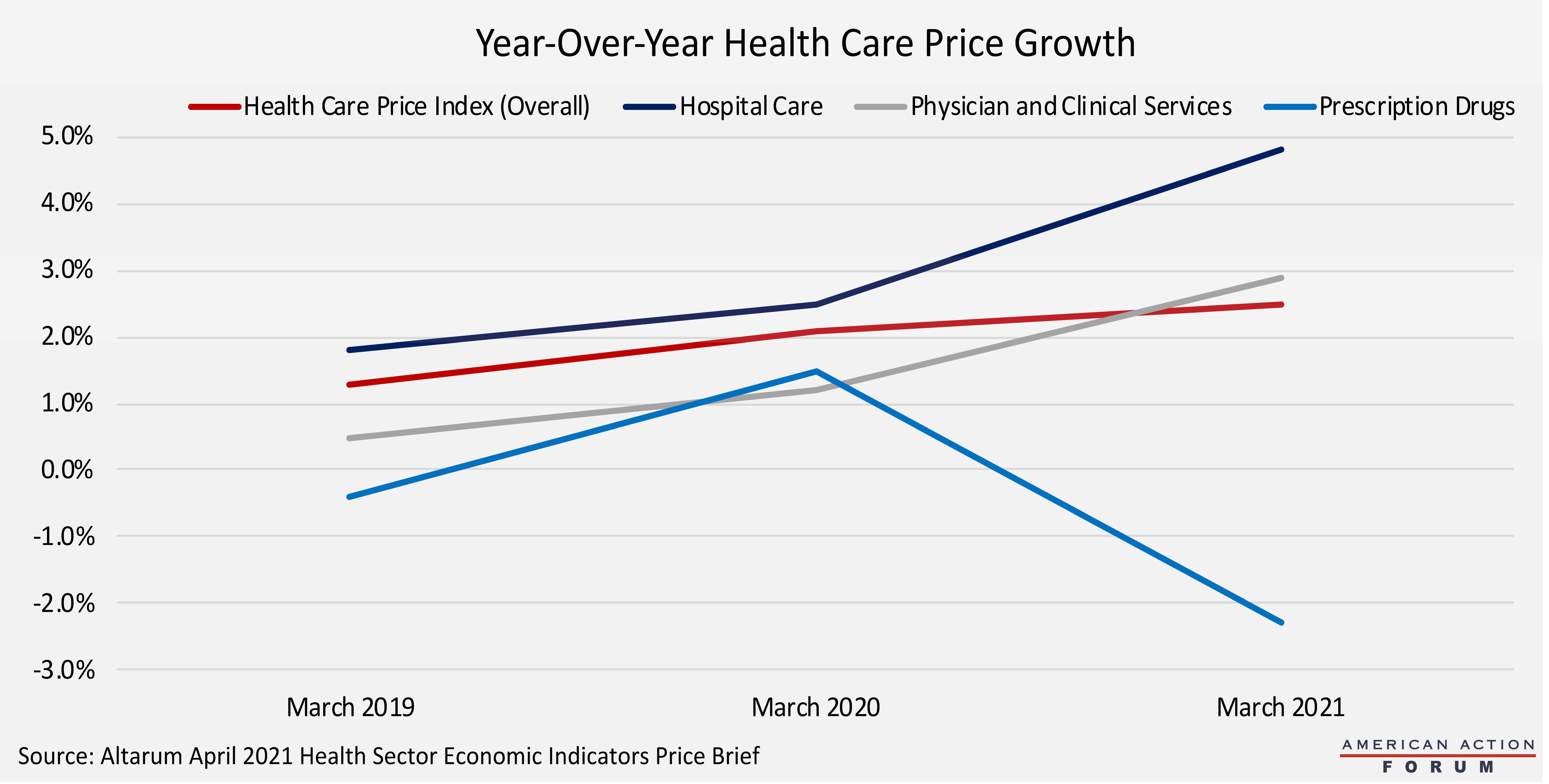
Over the past three years, overall health care prices have gradually increased, rising 1.3 percent between March 2018 and March 2019 and 2.5 percent from March 2020 to March of this year. There are, however, noticeably different rates of growth across the various health care product and service lines.
For example, hospital prices grew 1.8 percent and 2.5 percent year-over-year by March 2019 and March 2020, respectively, before jumping 4.8 percent by March 2021. Prices for physician and clinical services followed the same trend, but at a slower rate, rising 0.5 percent, 1.2 percent, and 2.9 percent in each of the past three years.
Prescription drug prices, on the other hand, have declined in two of the past three years: down 0.4 percent from March 2018 to 2019, rising just 1.5 percent by March 2020, and declining 2.3 percent by March 2021. In fact, March was the sixth straight month that prescription drug prices showed a year-over-year decline. These figures indicate that health care price growth is not being driven by prescription drug prices, but rather by ever-rising hospital prices, primarily, particularly given that hospital care accounts for nearly 40 percent of all health care expenditures while prescription drugs account for less than 20 percent.
Title I of H.R. 3
Title I of H.R. 3 would require the Secretary of Health and Human Services (HHS) to enter into a binding negotiation process with the manufacturers of at least 25 branded drugs, and up to 250 drugs, each year to set the Maximum Fair Price (MFP) for each drug for all third-party payers. As a starting point for the negotiation, the Secretary would establish a ceiling price of 120 percent of the volume-weighted average price of the drug in Australia, Canada, France, Germany, Japan, and the United Kingdom, or the Average International Market (AIM) price. Once the negotiations conclude and the new MFP is established, manufacturers would be prohibited from increasing their price above the rate of inflation. If the Secretary concludes that a manufacturer is not negotiating in good faith, the drug can be subjected to a tax equal to 95 percent of its revenue, in effect guaranteeing that the drug would be withdrawn from the domestic market.
It is worth emphasizing the sweeping nature of the proposed reforms. The MFP does not just apply to Medicare Part D, or Medicare as a whole, or even just federal programs. It would apply to all third-party payers, including plans operating under the Employee Retirement Income Security Act (ERISA).
The provisions are a uniquely undesirable combination of government “negotiation” and international reference pricing. Let us discuss each in turn.
Government Negotiation
Government negotiation has figured strongly in the debate over the structure of the Medicare Part D program. Direct negotiation by the Secretary of HHS has been expressly forbidden in the Part D statute. Yet the program nevertheless sees aggressive negotiation over the prices of medications between Part D plan sponsors and drug manufacturers. This competitive process is the key factor in the program’s success to date.
Today, Part D beneficiaries have access to 27 different plans, on average, enabling individuals to choose a plan that is tailored to their needs.[28] Because there are a number of plan options for beneficiaries, individual plans have the ability to use preferential tiering strategies to negotiate discounts for specific drugs. If a beneficiary requires or desires a specific medication that is not on the preferred formulary (or covered at all) for one plan, they can choose to sign up for a different plan that provides the medication at a more desirable price.
If the government, however, were to seek to negotiate the prices of specific drugs, the system would break down. Plans have leverage to drive discounts because they can restrict or deny access to specific medications or offer the medication in ways that make it more desirable to their beneficiaries. For the federal government to undertake this kind of negotiation, there would need to be a single federal formulary. In other words, the Secretary would have to be willing to say no to many treatments on behalf of all beneficiaries in order to drive discounts system-wide. Beneficiaries’ choices would drop from 27 plans to 1. Further, beneficiaries would no longer be able to shop for the plan that is best for them; rather, they would have to simply hope the government was able to negotiate a good deal for the drug(s) they need. Policymakers and the American public have long been reticent to make that trade off.
The Congressional Budget Office (CBO) has repeatedly held that in absence of a willingness to deny coverage for specific medications, the Secretary would not have the leverage necessary to drive any savings to the Part D program.[29] In short, given these constraints, direct negotiation of drug prices by the secretary would not work. H.R. 3 “solves” this problem by giving the Secretary access to a draconian tax that would effectively preclude access to the drug. This leverage, not negotiation, is the source of any projected savings.
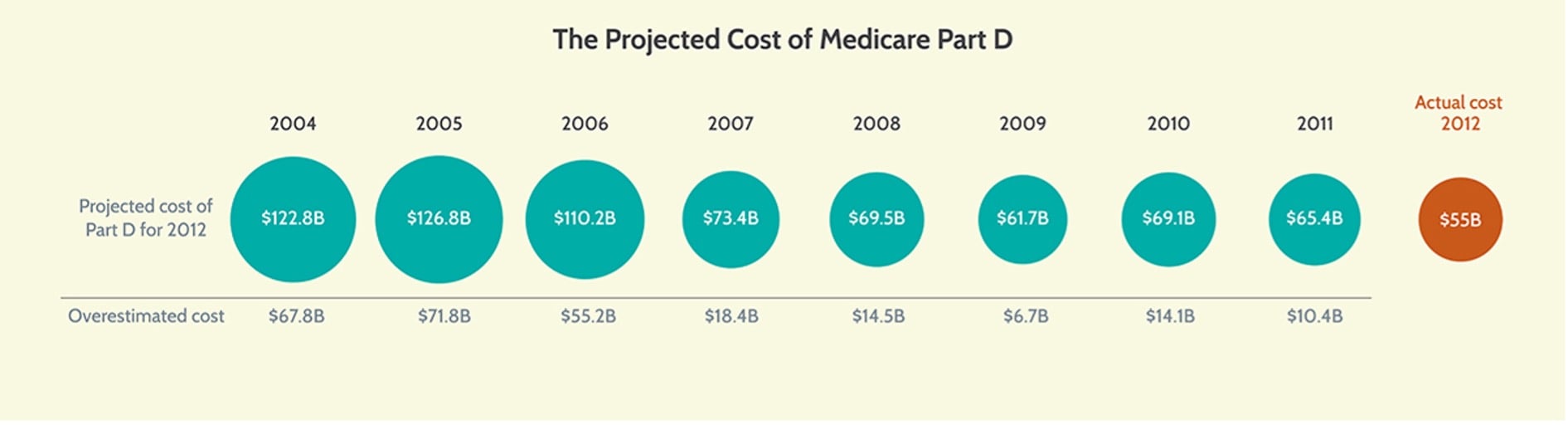
The current reliance on genuine, private negotiation has worked incredibly well. As demonstrated in the following infographic, total program expenditures came in far lower than initial CBO projections. Part D’s 10-year cost (starting in 2006) was projected in 2004 to be $957.3 billion, after the Medicare Modernization Act was passed but before the program started. By 2011, the combination of five years of actual data and five years of projections totaled $499.4 billion, for a cost under-run of $457.9 billion, or about 48 percent. The last CBO forecast for 2012 Part D spending made prior to implementation was in 2005, and the projected 2012 spending in that year was $126.8 billion. After the bids came in for 2006, the 2012 forecast was reduced to $110.2 billion. In all but one of the next six years, the forecast for 2012 was reduced further. The actual amount was $55.0 billion – about 57 percent lower than the original pre-implementation forecast.[30]
International Reference Pricing
As a starting point to the process outlined in Title I, the Secretary would establish a ceiling price of 120 percent of the volume-weighted average price of the drug in Australia, Canada, France, Germany, Japan, and the United Kingdom, or the Average International Market (AIM) price. This is a form of international reference pricing, also employed in the Trump Administration’s regulatory efforts to set an International Pricing Index (IPI) or a Most-Favored Nation (MFN) price in Medicare.
While the objective of reducing the cost of drugs and increasing Americans’ access to necessary medicines is laudable, such a policy could result in significant undesirable repercussions. The most likely consequences are restricted access to existing medicines and reduced innovation for future advancements and new medicines; cost-shifting to the private sector insurance markets; an undermining of the administration’s goal to move to value-based payments; and harm to U.S. trade objectives.
To see the implications, consider the IPI proposal. The 14 countries that CMS proposed referencing in the IPI model, on average, have access to only 48 percent of the new drugs developed in the past eight years, and it took an average of 16 months after their initial global launch for those drugs to become available in those 14 countries. The United States, on the other hand, has gained access to 89 percent of new medicines within three months.[31]
Adopting the non-market prices of other countries, and thus the punitive and authoritative policies used to obtain those prices, will likely also mean adopting for American patients similar levels of restricted access to new medicines as experienced in other countries. Worse yet, this demo may result in new medicines never being developed in the first place. Operation Warp Speed, undertaken by the Trump Administration, has been widely praised for accelerating the development COVID-19 vaccinees and therapies, saving lives and allowing for a faster economic recovery. But while Operation Warp Speed involved substantial federal investment, the effort was only possible because of years of industry research and development prior to the pandemic.[32] In a world where policies like those in H.R. 3 had been in place in preceding years, research may not have progressed far enough for an effort like Operation Warp Speed to be effective.
Americans highly value their access to and choice of new treatment options. The reduced innovation that will likely occur as a consequence of the reduced manufacturer revenues that will result from this model will have significant ramifications. Further, referencing the prices paid for drugs in countries that do not adequately reflect the value of medicines is inconsistent with adopting a value-based payment system.
Finally, this model will undermine American trade policy, which may have repercussions far beyond the pharmaceutical industry. The United States should instead work to strengthen intellectual property rights in other countries and fight compulsory licensing in trade agreements to end the coercive practices that allow countries to force manufacturers to provide their drug for less than it’s worth; this is the only way to get other countries to pay more so that we may hopefully pay less without risking reduced innovation.
Reforms to Medicare Part D
Title III of H.R. 3 contains reforms to Medicare Part D. A reform of this type would be a valuable improvement in Part D, if the parameters are set appropriately, and would place downward pressure on drug prices more generally.
Reasons for Reform
One reason for reform is to rein in rising reinsurance costs. At the program’s outset in 2006, reinsurance costs accounted for 26 percent of the government’s overall Medicare Part D subsidy. By 2019, reinsurance costs had climbed to 80 percent of the program’s basic subsidy, at $46.3 billion.[33] This shift has dramatically increased taxpayers’ financial exposure, putting the program on an unsustainable path. Between 2007 and 2017, premium subsidies decreased by 1.8 percent per year while reinsurance payments increased by 16.7 percent per year, on average.[34]
While rising drug spending itself is, of course, partially to blame for the increasing reinsurance costs, structural changes made to the program are also significant contributors. The Patient Protection and Affordable Care Act included two provisions that made significant changes to the Part D program: the Coverage Gap Discount Program—otherwise known as “closing the coverage gap” (or the “donut hole”)—and a temporary slowing of the catastrophic threshold’s growth rate.
The Coverage Gap Discount Program required drug manufacturers to pay 50 percent of the costs of any drug a beneficiary takes while he or she is in the coverage-gap portion of the benefit phases. These manufacturer payments count toward the beneficiary’s true out-of-pocket (TrOOP) cost calculation, which in turn determines when the beneficiary moves from one phase of the benefit to the next. Including manufacturer’s payments in this calculation results in beneficiaries moving through the benefit phases and into the catastrophic phase much more quickly than they otherwise would.
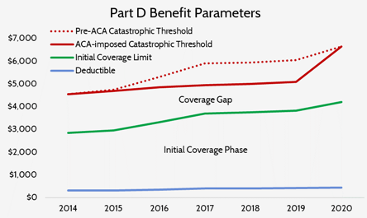
The temporary slowdown in the growth rate of the catastrophic threshold also led to more beneficiaries reaching the catastrophic phase and doing so after less spending than they would have absent this change, as can be seen below.
The growth rate of the catastrophic coverage threshold has now returned to normal, but a provision included in the Balanced Budget Act of 2018 (BBA) exacerbates the problem of rising reinsurance expenditures: The BBA increased manufacturer liability in the coverage gap to 70 percent while decreasing insurer liability to 5 percent.
The increase in manufacturer liability exacerbates the existing TrOOP problem. This change resulted in an unprecedented increase in the number of beneficiaries reaching the catastrophic phase, driven largely by beneficiaries not receiving the low-income subsidy (LIS). In 2019, 4.3 million individuals reached the catastrophic phase, with a 33 percent increase among non-LIS beneficiaries from the year prior.[35]
Further, the decrease in insurer liability significantly diminishes insurers’ incentive to control costs for high-cost enrollees. Insurers in 2019 were at risk for only 38.8 percent of the benefit costs, down from the expected 75 percent rate in 2007.[36] In fact, the BBA reduced insurer liability so substantially that for a beneficiary who reaches the catastrophic threshold in 2020 taking exclusively brand-name or biosimilar drugs, the insurer’s cost at this point ($2,974) is about $700 less than the drug manufacturer’s ($3,698).
A second issue is the financial burden on high-cost enrollees. The financial burden for enrollees reaching the catastrophic phase can be quite significant. In 2010, 2.4 million beneficiaries reached the catastrophic phase, and 33,000 beneficiaries did so after filling a single prescription.[37] In 2019, more than 4.3 million beneficiaries reached the catastrophic phase and more than 483,000 did so after a single prescription fill, up more than 100,000 just two years prior.[38] These beneficiaries had average spending of $26,482.[39] Beneficiaries who do not receive the LIS are responsible for 5 percent of all costs in the catastrophic phase, meaning the average non-LIS high-cost enrollee incurred more than $2,100 in OOP costs in 2019, and that figure will increase each year, particularly as more expensive specialty medicines are developed. As of 2019, specialty drugs account for 25 percent of Part D spending, approximately four times more than in 2010.[40]
The larger reason for reform is the perverse incentives for high-priced drugs. The structure of the current benefit design, as well as the pricing incentives in the broader drug market, encourage insurers to prefer coverage of high-price, high-rebate drugs, a preference that also leads to more beneficiaries reaching the catastrophic phase. Because of the BBA’s changes and the insurers’ varying liability for brand-name products (5 percent) versus generics (75 percent), it is now in insurers’ financial interest to prefer a brand-name drug over a generic unless the generic is more than 15 times less expensive than the brand-name product.[41] This incentive may depress demand for generics, which could discourage generic development, harming the broader market. Further, pharmacy benefit managers (PBMs)—working on insurers’ behalf—have created a business model in which their revenue is largely tied to the amount of rebates they are able to obtain from drug manufacturers.[42] Drug manufacturers provide greater rebates in order to secure preferred status on a drug plan’s formulary, which increases sales of the preferred drug. The easiest way to provide a larger rebate is to raise the price of the drug before the rebate (list price). Insurers benefit from greater rebates because these rebates, most often paid after the point of sale, can be used to reduce next year’s premiums—the most important factor beneficiaries consider when deciding in which plan to enroll.
The mandatory discounts that Part D requires of drug manufacturers in the coverage gap also encourage higher drug prices. Because the coverage gap is in the middle of the benefit structure, only a finite amount of spending is subject to the discount, resulting in a maximum discount that manufacturers will owe.[43] Under current law, the maximum discount a drug manufacturer will have to pay in 2022 is $4,136. Any manufacturer with a drug costing $10,399 or more will have to pay this discount. For a drug costing $10,399, this discount represents a discount of 40 percent. For a drug costing $50,000, this maximum discount will only equal 8.3 percent of the drug’s price. Thus, the current structure is more punitive to manufacturers of lower-cost drugs and encourages higher prices.
Further, patients who pay coinsurance based on the list price are substantially worse off when list prices rise. Also, because the rebates are used to reduce premiums for everyone, rather than simply reducing the high OOP costs paid by the patients taking the drug for which the rebate was paid, the beneficiaries with higher costs end up subsidizing those with lower costs—the opposite of how insurance is supposed to work. Taxpayers, who subsidize 75 percent of the program, are also worse off when program costs increase. Thus, higher-price, high-rebate drugs are beneficial to each of the industry stakeholders but increase costs for both patients and taxpayers.
Redesigning the Benefit to Realign Incentives
One possible reform to address these various problems would involve reconfiguring the liabilities within the Part D structure. These changes include placing a true cap on beneficiary OOP expenditures, eliminating the coverage gap phase entirely and instead requiring drug manufacturers to pay rebates during the catastrophic phase, reducing the federal government’s reinsurance rate, and increasing plans’ liability in the catastrophic phase.[44]
Requiring drug manufacturers to pay discounts in the catastrophic phase ensures that the amount they owe increases along with the drug’s price, discouraging both high launch prices and price increases. The current structure—which caps manufacturer liability as described above—results in the mandatory discount being more punitive for lower-cost drugs, thus encouraging higher prices.
Increasing insurer’s liability throughout the benefit, and particularly in the catastrophic phase, increases insurers’ incentive to control costs and reduce the use of high-cost, high-rebate drugs.
Reducing the government’s reinsurance liability reduces taxpayers’ exposure to rising costs.
Establishing an OOP cap provides beneficiaries with financial protection, assuring them they will not face open-ended OOP costs. More than a million beneficiaries could save hundreds or thousands of dollars each year.
While Title III of H.R. would make all of these changes, analysis by the American Action Forum finds that the specific parameters —the level of the OOP cap, the discount rate required of drug manufacturers, and the liability placed on insurers—will not yield optimal outcomes.[45] The parameters established by H.R. 19—particularly the slightly higher OOP cap, reduced beneficiary cost-sharing below the OOP cap, and uniform insurer liability for generics and brand-name medicines—are more likely to do so.
The lower OOP cap established in H.R. 3 should yield greater OOP savings for the minority of beneficiaries who reach the cap, but it will also result in greater federal reinsurance costs, relative to H.R. 19. Because most beneficiaries never reach $2,000 in OOP spending, more will benefit from the reduced cost-sharing below the catastrophic phase provided by H.R. 19, yielding greater total OOP savings for beneficiaries.
Further, because insurer liability under H.R. 3—both above and below the catastrophic phase—would be lower for brand-name drugs than generics, this legislation would maintain the current perverse incentives for plans to favor more expensive brand-name drugs over cheaper generic options, which increases costs for other stakeholders. H.R. 19, on the other hand, provides a uniform liability for insurers regardless of the type of drug, eliminating this preference, which should encourage use of less expensive medicines.
Conclusion
Fundamentally, there is no broad prescription-drug pricing crisis. Indeed, in most instances, things are working just fine. Rather, what we face are more nuanced challenges. For example, the price of specialty drugs and biologics, which are expensive to develop and manufacture and frequently treat a limited population, are very high. In these instances, particularly with oncology drugs, it is important to make sure that the cost of the treatments correlates to the value. Remember that the goal should not be low cost, but rather high value. It is easy to have low-cost drugs; they, however, may not do much good. Conversely, it might make sense to spend more for a drug if its therapeutic benefits are high enough.
The policies contained in Title I of H.R. 3 will stifle the kind of innovation that has made the United States the location of the most advanced medical therapy on the globe. As CBO dryly put it: “The lower prices under the bill would immediately lower current and expected future revenues for drug manufacturers, change manufacturers’ incentives, and have broad effects on the drug market.”
In contrast, the reforms of Medicare Part D are similar to the proposal first put forward by the American Action Forum in 2018 and were included in bills from all sides in both the House and the Senate. As noted above, the preferred formulation is contained in H.R. 19. The basic ideas are to provide beneficiaries an out-of-pocket cap, reduce the government’s reinsurance liability in the catastrophic phase, and requiring drug manufacturers to pay a share of the costs incurred in the catastrophic phase. The latter would increase the incentive for the manufacturer and insurer to negotiate prices that keep people out of the catastrophic phase of their insurance policy. If something “must” be done about drug prices, this would appear to be the most promising legislative route.
In either event, the Congressional Budget Office has indicated that the reforms will generate budget savings. Those savings should not be used to finance a broad expansion of government spending. Instead, they out to be used to extend the financial life of the Medicare program, or reduce overall spending.
Notes
[1] https://www.healthsystemtracker.org/brief/payments-for-cost-sharing-increasing-rapidly-over-time/
[2] https://www.americanactionforum.org/research/understanding-pharmaceutical-drug-costs/
[3] https://www.americanactionforum.org/research/understanding-pharmaceutical-drug-costs/
[4] https://www.cbo.gov/system/files/2019-05/55151-SupplementalMaterial.pdf
[5] https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html.
[6] https://altarum.org/sites/default/files/uploaded-publication-files/SHSS-Spending-Brief_April_2021.pdf
[7] https://altarum.org/sites/default/files/uploaded-publication-files/SHSS-Price-Brief_March_2021.pdf
[8] https://www.iqvia.com/institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022
[9] https://www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm
[10] http://www.partnershipforsolutions.org/DMS/files/chronicbook2004.pdf
[11] http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/prevention-chronic-care/decision/mcc/mccchartbook.pdf
[12] https://www.chronicdisease.org/page/whyweneedph2imphc
[13] https://csdd.tufts.edu/csddnews/2018/3/9/march-2016-tufts-csdd-rd-cost-study
[14] https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2015/research-and-development-in-the-pharmaceutical-sector_health_glance-2015-70-en
[15] http://www.fdareview.org/approval_process.shtml
[16] http://www.fdareview.org/approval_process.shtml
[17] https://www.pcmanet.org/pcma-cardstack/what-is-a-specialty-drug/, https://cvshealth.com/thought-leadership/whats-special-about-specialty
[18] https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/index.html.
[19] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023
[20] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us
[21] https://altarum.org/sites/default/files/uploaded-publication-files/SHSS-Price-Brief_January_2019.pdf
[22] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us
[23] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us
[24] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us
[25] https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicine-use-and-spending-in-the-us-a-review-of-2017-and-outlook-to-2022.pdf
[26] https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us
[27] https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicine-use-and-spending-in-the-us-a-review-of-2017-and-outlook-to-2022
[28] https://www.kff.org/medicare/press-release/people-on-medicare-will-be-able-to-choose-among-24-medicare-advantage-plans-and-27-medicare-part-d-drug-plans-on-average-during-the-open-enrollment-period-for-2019-new-analyses-find/
[29] https://www.cbo.gov/sites/default/files/108th-congress-2003-2004/reports/03-03-wyden.pdf
[30] https://www.americanactionforum.org/research/competition-and-the-medicare-part-d-program/#_ftn7
[31] https://www.americanactionforum.org/comments-for-record/comments-to-cms-on-proposed-international-pricing-index-for-medicare-part-b-drugs/
[32] See the discussion here: https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
[33] http://www.medpac.gov/docs/default-source/default-document-library/ma_vip_public_jan_2020.pdf?sfvrsn=0
[34] http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch14_sec.pdf?sfvrsn=0
[35] http://www.medpac.gov/docs/default-source/reports/mar21_medpac_report_ch13_sec.pdf?sfvrsn=0
[36] http://www.medpac.gov/docs/default-source/reports/mar21_medpac_report_ch13_sec.pdf?sfvrsn=0
[37] http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch14_sec.pdf?sfvrsn=0
[38] http://www.medpac.gov/docs/default-source/data-book/july2020_databook_sec10_sec.pdf?sfvrsn=0
[39] http://www.medpac.gov/docs/default-source/reports/mar21_medpac_report_ch13_sec.pdf?sfvrsn=0
[40] http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch14_sec.pdf?sfvrsn=0
[41] For low-income subsidy (LIS) enrollees, the distortion is even worse because plans have no liability for such beneficiaries in the coverage gap.
[42] https://www.americanactionforum.org/research/primer-prescription-drug-prices-discounts-fees-effects-part-d/
[43] https://www.americanactionforum.org/insight/the-impact-of-shifting-rebates-to-catastrophic-coverage-in-medicare-part-d/
[44] https://www.americanactionforum.org/research/redesigning-medicare-part-d-realign-incentives-1/
[45] https://www.americanactionforum.org/insight/analysis-of-the-competing-proposals-to-reform-medicare-part-d/